XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most stable one. It is a white
crystalline solid used for fluorinating purposes in electrochemical procedures and laboratories. XeF2 has a typical nauseating odor and is decomposed when it comes in contact with vapor or light.
It is vital to know it’s Lewis structure, hybridization and polarity to understand the chemical properties as well as molecular geometry of the compound. So lets now understand all the properties in detail.
Contents
Xef2 Lewis Structure
The Lewis structure of a given chemical compound is crucial for knowing all the physical properties and chemical properties. It is a pictorial representation of all the electrons participating in forming bonds. This structure helps in understanding the charges on the molecules of the compound. The electrons that participate in bond formation, as well as the ones that do not participate, are collectively known as valence electrons.
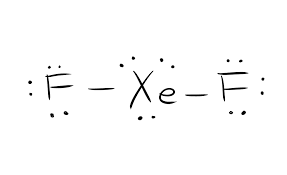
Electrons that take part in forming bonds are called bonding pairs of electrons. The ones that do not participate in bond formation are called lone pair of electrons. For distinguishing between the different types of electrons, both bonding and lone pairs of electrons are represented differently. The bond formation in the compound are represented as straight lines and the lone pairs are depicted as dots.
Lewis structure is based on the octet rule. This rule states that every molecule should have eight electrons in its outer shell of an atom to be stable. If there are more electrons than it, then that compound donates the electron. Whereas if there are less than eight electrons, the compound accepts the electrons from the other molecule to be stable.
So for this compound XeF2, there is one molecule of Xenon and two molecules of Fluorine. A single molecule of Xenon has eight electrons, and a Fluorine molecule has seven valence electrons.
Total number of valence electrons = No. of valence electrons for Xenon + No. of valence electrons for Fluorine
=8+ 7*2
=8+14
=22
The total number of valence electrons for XeF2=22.
Xef2 Hybridization

Hybridization of a given molecule is vital to understand the geometry of the molecule. During bond formation, two or more orbitals with different energy levels combine and make hybrid orbitals. In XeF2, the outer shell of Xenon has eight electrons out of which two electrons participate in bond formation.
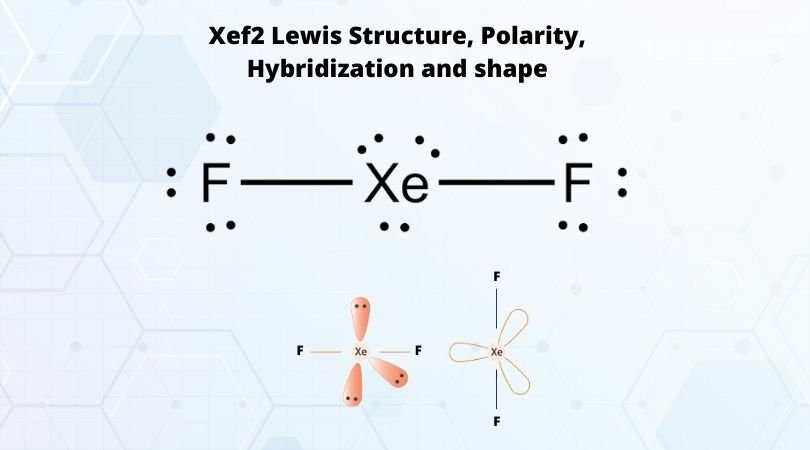
The ground state of the Xenon has 8 electrons arranged in s2 p6 orbitals. Whereas in XeF2, the Xe molecule has an excited state. The arrangement of the electrons of Xenon changes to s2 p5 d1 with two unpaired electrons. Hence the hybridization of the central atom Xe is sp3d. Thus the hybridization of XeF2 molecule is sp3d.
Xef2 Molecular Geometry

Generally, the Lewis structure is helpful to understand the molecular geometry of any given chemical compound. But as Xenon does not form bonds easily, this compound is an exceptional case. The molecular geometry of Xenon Difluoride can be understood by knowing the VSEPR theory. This theory is based on the steric number of the central atom and the valence electrons of the compound. VSEPR is an abbreviation for Valence Shell Electron Pair repulsion theory.
Here the steric number for the central Xenon atom is 5. This means that a single molecule of Xenon can form bonds with five molecules. But here in XeF2, it is forming bonds with two Fluorine atoms only. For Xenon, two electrons out of eight form bonds with the fluorine atoms. These six electrons are now the non-bonding electrons. These three lone pairs of electrons spread out in an arrangement that is on the equatorial position to the bonded pairs of electrons.
The shape of the molecule should be trigonal bipyramidal as per the hybridization, but it is not. XeF2 is a linear molecule due to the arrangement of fluorine atoms and the lone pairs of electrons in the symmetric arrangement.
Bond Angle
Now that we know the molecular geometry of Xenon Difluoride molecule, the bond angle can be understood easily. There are two pairs of bonded electrons and three lone pairs of electrons. The lone pairs are on the equatorial position to the bonded pairs. The bond angle between the two pairs bonded with the central atom is 180 degrees, which makes the molecular geometry of XeF2 linear.
Xef2 Polarity

The polarity of any given molecule depends on the molecular geometry and the hybridization of the compound. In XeF2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom Xenon in the middle. There is no net dipole moment in the compound due to the arrangement of the valence electrons in symmetry. Hence Xenon Difluoride is nonpolar as there is no polarity observed in the molecule.
Concluding Remarks
To summarize the article, it can be concluded that XeF2 has 22 valence electrons, out of which there are three lone pairs of electrons. Its hybridization is sp3d. According to the VSEPR theory, The molecular geometry of the molecule is linear. The bond angle of F-Xe-F is 180 degrees. As there are fluorine molecules on both the side of the central atom, there is no dipole moment and hence there is no polarity. XeF2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons.

I so much appreciate this,through this i could deferentiate compound from molecule,and knowing the lewis structure/hybridization.moreso, the molecule geometory and it’s polarity/non polarity.i really got a total sumary of this article.By:Janice powell.