Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts.
Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules.
SF6 cannot be used immediately after synthesis. It needs to be purified to get rid of all reactive fluorides. After that, it needs to go through pyrolysis.
Here in this blog post, we will learn the Lewis Structure of SF6 and its Bond angles, Molecular geometry and shape that can help us understand the physical properties of this molecule.
| Name of molecule | Sulphur Hexafluoride ( SF6) |
| No of Valence Electrons in the molecule | 48 |
| Hybridization of SF6 | sp3d2 hybridization |
| Bond Angles | 90 degrees |
| Molecular Geometry of SF6 | Octahedral |
Contents
SF6 Valence Electrons
To determine the Lewis Structure of any molecule, we first need to know the total number of valence electrons. Here we will find out the total number of valence electrons for SF6 by adding the valence electrons for both Sulfur and Fluorine atoms.
Total number of valence electrons in SF6 – Valence electrons of Sulfur + Valence electrons of Fluorine
Sulfur has six valence electrons.
Fluorine has seven valence electrons, but as there are six Fluorine atoms in this molecule, we will multiply this number by 6.
= 6 + 7*6
= 6 + 42
= 48 valence electrons
Thus SF6 has 48 valence electrons that will help us draw the Lewis Dot Structure of SF6.
SF6 Lewis Structure
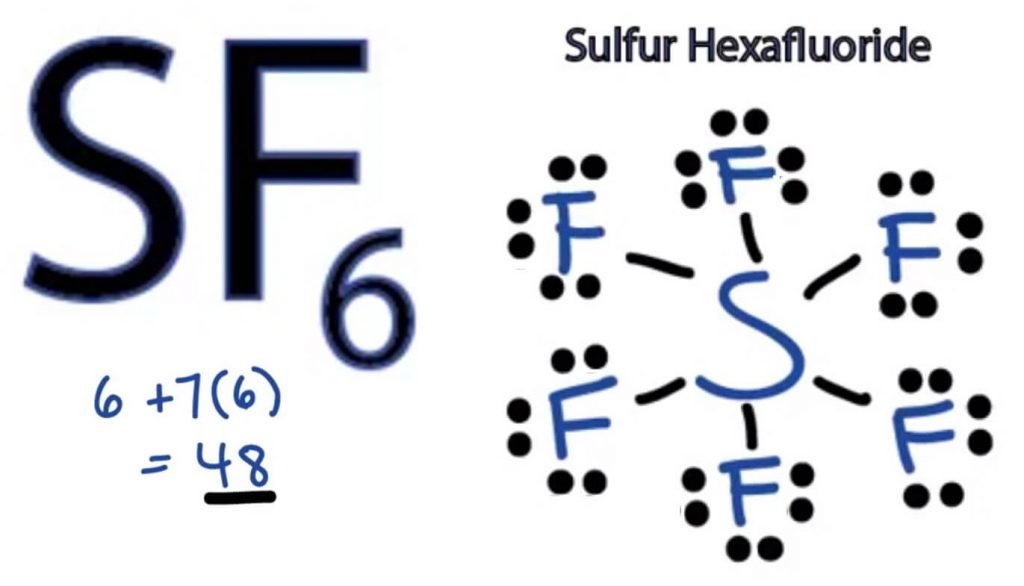
The Lewis Dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons. This structure helps us know the bond formations in the molecule and the arrangement of electrons in it.
Sulphur atom will take the central position as it is less electronegative than Fluorine. So place it in the centre and all Fluorine atoms around it like this:
Fluorine atom needs only one valence electron to complete its octet. As every atom follows the octet rule to attain a stable structure, the Fluorine atom will share one valence electron of the Sulphur atom. Thus, Sulphur will share six of its valence electrons with all the fluorine atoms that result in forming six single bonds between S and F.
In Lewis Structure, we show the bonds in the structure by drawing a straight line between two atoms. So all these bonds will take up 12 valence electrons out of 48.
Place all the remaining valence electrons around the Fluorine atoms and check if the octets of all the fluorine atoms are complete.
Once you do that, you will see valence electrons in the outer shells of all Fluorine atoms, but Sulphur has more than 8 electrons in its outer shell. This is because it is an exception to the octet role and can expand its orbital to accommodate more electrons.
Hence, this is the right Lewis structure of SF6.
SF6 Hybridization
Now that we know the Lewis Structure of SF6, we can now determine the atoms’ hybridization in the molecule. Here as Sulphur is sharing its electrons with the Fluorine atoms, we will look at its hybridization.
The electronic configuration of SF6 in its ground state is 3s23p4. But when it shares electrons and is in the excited state the electron pairs in both 3s and 3p orbitals get unpaired. These electrons move to fill the higher vacant 3d orbitals. As a result, six hybrid orbitals are formed ( one of 3s, three of 3p, and two 3d). These hybrid orbitals are the ones that accommodate the shared electrons. These orbitals overlap with the 2p orbitals of the fluorine atom when Sulfur and Fluorine atoms form bonds. These six orbitals are in the six directions of the octahedron shape.
Hence, Sulfur Hexafluoride has sp3d2 hybridization.
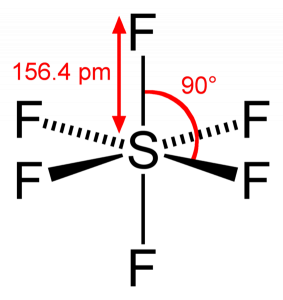
SF6 Bond angle
As Sulphur shares its valence electrons with 6 Fluorine atoms, we can see that all six electrons of the Sulphur atom are shared to form bonds. The bond angle of F-S-F is 90 degrees.
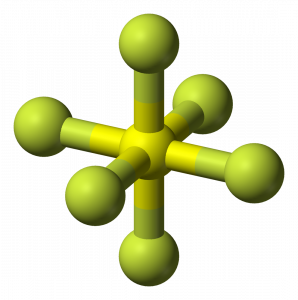
SF6 Molecular Geometry
When we look at Sulphur Hexafluoride molecule, Sulphur is in the central position with the fluorine atoms arranged symmetrically around it. The atoms are placed in the octahedral pattern, which makes the molecular geometry of SF6 is octahedral.
SF6 Shape
Looking at the molecular geometry of the molecule, we can say that the SF6 molecule has an octahedral shape as it has eight sides. However, the central atom bonds with six Fluorine atoms, the shape of SF6 is octahedral.
Is SF6 polar or nonpolar?
SF6 is a nonpolar molecule. This is because the VSEPR theory says that when six fluorine atoms are arranged symmetrically around the sulfur atom, the bond dipoles are cancelled. As a result, it is a nonpolar molecule.
It also has the same properties as non-polar molecules such as being non-soluble in water and being soluble in non-polar organic solvents.
Concluding Remarks
To summarize this article we can say that in the Lewis dot structure of SF6, all the valence electrons are used up which results in forming six single bonds between S-F with no lone pairs of electrons.
The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees.
The molecular geometry of SF6 is octahedral and it is a nonpolar molecule.
This may, or may not interest you. I have a question. Is the SF6 molecule capable of bonding with a virus? Corona virus to be specific? I am struck by the ultra-covalent properties of this molecule. Other concerns are the presence of it around the earth, used as an insulator. My central worry is that we are battling a pathogen that inadvertently may have been mutated from its original form by a benign, but incredibly bond-friendly man made substance. Is it possible? And if possible, would the use of good old sulfa-type antibiotics a proximate solution for those a worsened state of morbidity? I am not a doctor. I have been trained in Medical coding, and we had to be educated in pathology, definitions, anatomy, and the ICD’s in order to graduate. I cannot help but wonder that some of our pathogenic enemies (to the human condition) are created by us, or at the least inadvertently enabled by us.
Respectfully,
B. A. A. Khan
That was too easy to understand 🙂
Thank you for the kind words.