Hydrazine is an inorganic pnictogen with the chemical formula N2H4. It is a colorless liquid with an Ammonia-like odor.

Hydrazine is highly flammable and toxic to human beings, producing seizure-like symptoms. It is primarily used as a foaming agent (think foam packaging) but also finds application in pesticides, airbags, pharmaceuticals, and rocket propulsion. It is also a potent reducing agent that undergoes explosive hypergolic reactions to power rockets. This inherent property also dictates its behavior as an oxygen scavenger, as it reacts with metal oxides to significantly reverse corrosion effects.
The creation of the single-bonded Nitrogen molecule is a critical step in producing Hydrazine. The Raschig process is most commonly employed to manufacture Hydrazine on a large scale. Ammonia (or Urea) is oxidized in the presence of Sodium Hypochlorite to form Hydrogen Chloride and Hydrazine.
![]()
![]()
Nitrogen and Oxygen are released when Hydrazine undergoes Oxygen-induced combustion. As a potent reducing agent, it reacts with metal salts and oxides to reverse corrosion effects. Hydrazine forms salts when treated with mineral acids. Hydrazine sulfate use is extensive in the pharmaceutical industry.
Let’s understand Hydrazine better. Some of its properties are given in the table below:
| Name of the molecule | Hydrazine (N2H4) |
| No. of valence electrons | (5 x 2) + (1 x 4) = 14 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 104.5° |
| Molecular Geometry of NH2- | Bent Molecular Geometry |
Contents
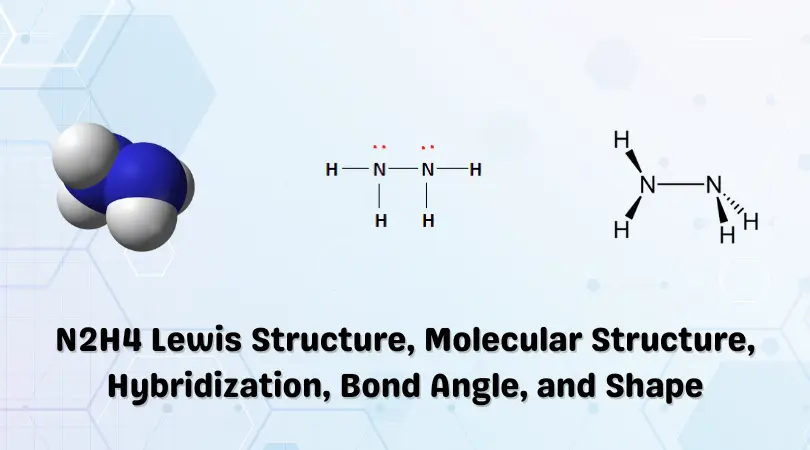
N2H4 Lewis Structure
Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. The simplified arrangement uses dots to represent electrons and gives a brief insight into various molecular properties such as chemical polarity, hybridization, and geometry.
Lewis structures are simple to draw and can be assembled in a few steps. The first step is to calculate the valence electrons present in the molecule.
Valence Electrons
Valency is an element’s combining power that allows it to form bond structures. Molecules can form single, double, or triple bonds based on valency.
In contrast, valence electrons are those electrons that lie in the outermost shell of the atom. Here, the force of attraction from the nucleus on these electrons is weak. Thus, valence electrons can break free easily during bond formation or exchange.
Each atom in the molecule contributes a set number of valence electrons depending upon their atomic number and position on the periodic table. These electrons are ‘pooled together’ to assemble a molecule’s Lewis structure.
Hydrazine comprises four Hydrogen atoms and two nitrogen atoms.
Nitrogen is in group 5 of the periodic table with the electronic configuration 1s22s22p3. Therefore, the two Nitrogen atoms in Hydrazine contribute 5 x 2 = 10 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the four Hydrogen atoms contribute 1 x 4 = 4 valence electrons.
Therefore, the total number of valence electrons present in Hydrazine [N2H4] is given by:
4[H] + 10[N] = 14 valence electrons
Lewis Structure Assembly
Step 1 in obtaining the Lewis structure of Hydrazine[N2H4], i.e., calculation of valence electrons, is now complete. There are a total of 14 valence electrons available.
The distribution of valence electrons in a Lewis structure is governed by the Octet rule, which states that elements from the main group in the periodic table (not transition metals/ inner-transition metals) form more stable compounds when 8 electrons are present in their valence shells or when their outer shells are filled. There are exceptions to the octet rule, but it can be assumed unless stated otherwise.
Now, the two Nitrogen atoms present are placed in the center, adjacent to each other. This will facilitate bond formation with the Hydrogen atoms. Next, the four Hydrogen atoms are placed around the central Nitrogen atoms, two on each side. To understand better, take a look at the figure below:

The valence electrons are now placed in between the atoms to indicate covalent bonds formed. Place two valence electrons in between the atoms as shown in the figure below:

The red dots represent the valence electrons. Ten valence electrons have been used so far. There are four valence electrons left. To determine where they are to be placed, we go back to the octet rule. With two electrons present near each Hydrogen, the outer shell requirements of the Hydrogen atoms have been fulfilled. Nitrogen atoms have six valence electrons each. This means that the four remaining valence electrons are to be attributed to the Nitrogen atoms. The arrangement is shown below:

All the outer shell requirements of the constituent atoms have been fulfilled. The final Lewis structure of Hydrazine is shown below:
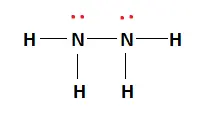
The black lines in the above figure indicate the covalent bond formed due to the sharing of electrons between the atoms. The red dots present above the Nitrogen atoms represent lone pairs of electrons. These valence electrons are unshared and do not participate in covalent bond formation.
N2H4 Hybridization
Molecular structure and bond formation can be better explained with hybridization in mind. The Hybrid orbitals formed to give a more accurate description of electron regions while also resulting in more stable bonds.
An easy way to determine the hybridization of an atom is to calculate the number of electron domains present near it. The bond between atoms (covalent bonds) and Lone pairs count as electron domains.
Two domains give us an sp hybridization. Three domains give us an sp2 hybridization and so on. In Hydrazine[N2H4], the central Nitrogen atom forms three covalent bonds with the adjacent Hydrogen and Nitrogen atoms. There is also a lone pair present. Therefore, three sigma bonds and a lone pair mean that the central Nitrogen atoms have an sp3 hybridization state.
N2H4 Bond Angles
According to the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), the lone pair on the Nitrogen and the electron regions on the Hydrogen atoms will repel each other resulting in bond angles of 109.5°.
N2H4 Molecular Geometry and Shape
As we discussed earlier, the Lewis structure of a compound gives insight into its molecular geometry and shape. From the Lewis structure, it can be observed that there are two symmetrical NH2 chains. The valence electrons on the Hydrogen atom and lone pairs present repel each other as much as possible to give the molecule a trigonal pyramidal shape.
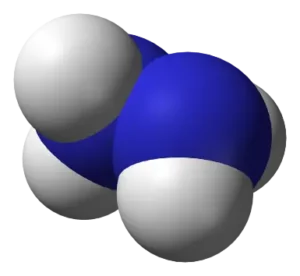
We can use the A-X-N method to confirm this.
‘A’ here represents the central Nitrogen atom. Therefore, ‘A’ = 1. Observe the right side of the symmetrical chain- the Nitrogen atom on the right will be considered the central atom.
‘X’ represents the number of atoms bonded to the central atom. In this case, a nitrogen atom and two hydrogen atoms are bonded to the central nitrogen atom.
Therefore, X =3.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1, and a single lone pair of electrons is attached to the central nitrogen atom.
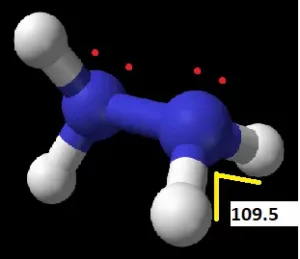
Therefore, that would give us an A-X-N notation of AX3N for the Hydrazine molecule[N2H4].
From the A-X-N table below, we can determine the molecular geometry for N2H4.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
From the above table, it can be observed that an AX3N arrangement corresponds to a Trigonal Pyramidal geometry.
Therefore, Hydrazine can be said to have a Trigonal Pyramidal molecular geometry. The two lone pairs and a steric number of 4 also tell us that the Hydrazine molecule has a tetrahedral electronic shape.
CONCLUDING REMARKS
Let’s quickly summarize the salient features of Hydrazine[N2H4]
- The N2H4 molecule comprises a symmetrical set of two adjacent NH2 groups. The single bond between the Nitrogen atoms is key here.
- Single bonds are formed between Nitrogen and Hydrogen. There are also two lone pairs attached to the Nitrogen atom.
- The hybridization of the central Nitrogen atom in Hydrazine is sp3.
N2H4 has a trigonal pyramidal molecular structure and a tetrahedral electronic shape. This results in bond angles of 109.5°.