Dinitrogen or nitrogen gas is a nonpolar molecule having no electric dipole moment. Wondering how? Well, this detailed blog post will help you understand it. The molecule comprises two Nitrogen atoms making it a diatomic molecule. Having a chemical formula of N2, nitrogen gas is one of the most abundant gases in our planet earth’s atmosphere. It is an odourless and colourless gas. This article on N2 gas will help you determine its polarity, Lewis structure and uses.
The nitrogen element is represented by using the element symbol N on the periodic table. For N2 gas, two atoms of Nitrogen bond together, forming a homonuclear atom. As the Nitrogen atom belongs to group 5A, it has five valence electrons in its outer shell. All the elements follow the octet rule, where each atom tries to attain stability by having eight valence electrons in its outer shell.
The Lewis dot structure for Nitrogen will be this:
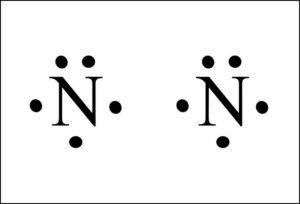
However, when two atoms of Nitrogen bind together, it has the following structure:

Here both these atoms share six valence electrons to form a triple bond. These electrons are shared equally, and both Nitrogen atoms have one lone pair of electrons.
N2 Polarity
Any molecule that exhibits polar ends in its structure is called a polar molecule. Such molecules have a partial positively charged and a partial negative charged end. This polarity is due to the net dipole moment in the molecule that can be determined by various factors such as
- Geometry of molecule
- Shape of the molecule
- The difference in electronegativities of atoms.
Geometry and shape of N2 molecule: In N2, both Nitrogen atoms form a bond to complete the octets. It is similar to other gases and elements such as O2, Cl2, Br2 and much more. However, the molecule has linear geometry as it is arranged in the same plane.
The difference in electronegativities of atoms in N2: While looking at the polarity of any molecule, we consider the difference in electronegativities of the atoms in the molecules because most of the time, these atoms are polyatomic heteronuclear molecules.
However, here N2 is made up of 2 Nitrogen atoms. The electronegativity value for the Nitrogen atom is 3.04, which will be the same for both atoms forming a bond in the molecule. And as there is no difference in electronegativities in this molecule, there will be no uneven distribution of charges.
Both the Nitrogen atoms will share the electrons equally, and as a result, there will be no partially charged regions formed in the molecule.
Net dipole moment in N2: As there is no dipole moment in the N2 molecule, the net dipole moment in the molecule is zero.
Summing up everything we stated above, we can say N2 or Nitrogen gas is a nonpolar molecule because there is no net dipole moment in the molecule as both the atoms are identical in nature. There is no difference in electronegativities in the atoms of this molecule.
Uses of N2 gas:
- Nitrogen gas is widely used in the food industry as it helps preserve and store food for a longer time.
- It is also used in the chemical industry for the production of fertilizers, dyes, chemicals, nitric acid, nylon and more. For use in such processes, N2 gas is first reacted with hydrogen, where it produces ammonia.
- Liquid Nitrogen is used in molecular gastronomy as well as refrigerant.
- Nitrogen is used in large quantities for annealing stainless steel.
- Liquid Nitrogen is also used for preserving and freezing eggs, sperms and other cells vital for research.
Nitrogen is also found in all living organisms as it is an integral part of amino acids, nucleic acids and the energy transfer molecule – ATP.
So, N2 polar or nonpolar?
Summarizing this blog post, we can say that N2 or Dinitrogen is a nonpolar molecule as there is no net dipole moment in the molecule given it is a diatomic molecule. Hence one can say that Nitrogen gas is nonpolar.




