The chemical formula NH2– represents the Azanide ion. Azanide is the IUPAC name of the ion; however, this nomenclature can be confusing. The derivatives of the NH2– ion are referred to as amides. The term ‘amide’ also refers to the –C(O)NR2, which is an organic functional group. To differentiate them, we must understand how these two Nitrogen-based compounds are formed.
Unlike its organic counterpart, the Azanide ion is a conjugate base of Ammonia. Deprotonation of Ammonia occurs when it reacts with strong bases or alkali metals. This leads to the formation of the Azanide anion.
Alkali metal derivatives (also known as alkali metal amides) such as Sodium Amide, Lithium Amide, and Potassium Amide are produced when liquid Ammonia is treated with strong bases. A schematic equation of this reaction is shown below:
2 M + 2 NH3 → 2 MNH2 + H2 (M = Li, Na, K)
Of these derivatives, Sodium Amide or Sodamide is used extensively in several chemical processes. As a strong base, it is used in organic synthesis and the drying of Ammonia.
Let’s understand the properties of the Azanide ion. Some of them are given in the table below:
| Name of the ion | Azanide (NH2–) |
| No. of valence electrons | (1 x 2) + 5 = 7 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 104.5° |
| Molecular Geometry of NH2– | Bent Molecular Geometry |
Contents
NH2– Valence Electrons
Valency is defined as the combining power an element possesses that allows it to form bond structures. This directly corresponds with their ability to form single, double, or triple bonds. In other words, it is the number that indicates how many bonds an element can form.
Valence electrons, on the other hand, are a different matter. They are a prerequisite for learning more about Lewis structures. Valence electrons are those electrons that lie in the outermost shell of the atom. Here, the force of attraction from the nucleus on these electrons is weak. Thus, valence electrons can break free easily during bond formation or exchange.
Each constituent atom contributes a specific number of valence electrons to the corresponding Lewis structures. Thus, these electrons help us conceive Lewis structures and act as their building blocks.
Let us find out the number of valence electrons available to us to get the Lewis Structure of the Azanide ion.
The Azanide ion comprises two Hydrogen atoms and one nitrogen atom. It also possesses a negative charge of -1 (since it is an anion).
Nitrogen is in group 5 of the periodic table with the electronic configuration 1s22s22p3.Therefore, the lone Nitrogen atom contributes 5 x 1 = 5 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the two Hydrogen atoms contribute 1 x 2 = 2 valence electrons.
Is that all? No. We must not forget the negative charge present. This negative charge of 1 contributes one valence electron as well.
Therefore, the total number of valence electrons in the Azanide ion [NH2–] is given by:
2[H] + 5[N] + 1[Neg-charge] = 8 valence electrons.
NH2– Lewis Structure
Lewis structures are schematic representations of the molecule. They help give insight into a host of molecular properties such as geometry and polarity.
To form Lewis structures, we must adhere to a few steps. The first step is to calculate the number of valence electrons available to us. These valence electrons are responsible for bond formation, among other things.
As discussed above, there are eight valence electrons available to us. Now, we must start arranging the constituent atoms to form a skeletal structure.
Nitrogen will act as the central atom and facilitate bond formation with the surrounding hydrogen atoms. Two valence electrons are used to form each basic singular covalent bond between the Nitrogen and Hydrogen atoms. This is shown in the figure below:

There are now four valence electrons available to use. The next step is to fulfill the octet requirements of the constituent atoms present in the compound. Hydrogen’s outer shell requirements have been fulfilled since each of the Hydrogen atoms has 2 electrons each.
Therefore, the four remaining valence electrons attach themselves to the central Nitrogen atom as lone pairs. This fulfills the Nitrogen atoms’ octet requirements. Therefore, the electronic arrangement and stable Lewis structure diagrams are shown below:

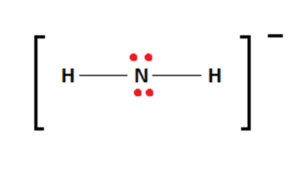
The brackets and negative sign over the structure are mandatory when ions are being represented.
NH2– Hybridization
The concept of orbitals provides a more accurate representation of electronic regions. This is in agreement with Heisenberg’s principle of uncertainty. According to the Valence Bond Theory, sometimes orbitals of equivalent energy fuse together to form new, hybrid orbitals when required. This process is known as hybridization.
An easy way to determine the hybridization is to calculate the number of electron domains present. The bond between atoms and Lone pairs counts as electron domains.
Two domains give us an sp hybridization. Three domains give us an sp2 hybridization and so on. In the case of the Azanide ion, there are two bonds and two lone pairs present, giving us 4 electron domains.
Therefore, the Azanide ion [NH2–] has a hybridization of sp3.
NH2– Bond Angles
In accordance with the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), the Hydrogen atoms will repel each other at bond angles of 109.5°. However, in addition to this, the repulsion due to the lone pairs present reduces the bond angle of the Azanide ion to 104.5°.
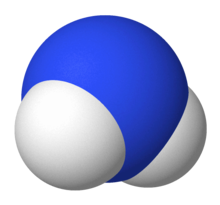
NH2– Molecular Geometry and Shape
As we discussed earlier, the Lewis structure of a compound helps determine its molecular geometry and shape. From the Lewis structure, we can observe that two Hydrogen atoms are surrounding the central Nitrogen atom. There are also two lone pairs.
As we visualize this, we must keep in mind the VSEPR theory of electron pair repulsion. The two Hydrogen atoms repel each other in the presence of lone pairs to give the Azanide ion a Bent molecular geometry.
We can use the A-X-N method to confirm this.
‘A’ here represents the central atom Nitrogen. Therefore, ‘A’ = 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, two Hydrogen atoms are bonded to the central nitrogen atom.
Therefore, X =2.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 2 is two lone pairs attached to the central nitrogen atom.
Therefore, that would give us AX2N2 for the Azanide ion (NH2–)
From the A-X-N table below, we can determine the molecular geometry for NH2–.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
From the above table, it can be observed that an AX2N2 arrangement corresponds to a Bent Molecular geometry.

Therefore, the Azanide ion (NH2–) has a bent molecular geometry. The two lone pairs and a steric number of 4 also tells us that the Azanide ion has a tetrahedral electronic shape.
Concluding Remarks
Let’s quickly summarize the salient features of NH2–
- The NH2– ion comprises a central Nitrogen atom bonded to two hydrogen atoms.
- Single bonds are formed between Nitrogen and Hydrogen. There are also two lone pairs attached to the Nitrogen atom.
- The hybridization of the Azanide ion is sp3.
- NH2– has a Bent molecular structure and a tetrahedral electronic shape. This results in bond angles of 104.5°.