It is vital to be well-versed with the different types of molecular shapes to understand the geometry of the molecules. These molecular shapes help understand several properties of the given compound, as the arrangement of the atoms in the complex depends on the number of electron pairs in the valence shell. This electron configuration helps to determine the molecular structure and to get a three-dimensional structure of these complexes.
One can use several theories to predict this molecular structure and understand the arrangements of the electrons in it. One of the commonly used models used to study the geometry of molecules is the Valence Shell Electron Pair (VSEPR) theory. This model is used widely to understand the covalent bonds between the nonmetal compounds in the complex. Whereas when it comes to understanding the structure of metal ions and compounds, the model of Cyrstal Field Theory ( CFT ) is used. This model aids in better understanding the metal-ligand bonds and the geometry of transition molecules.
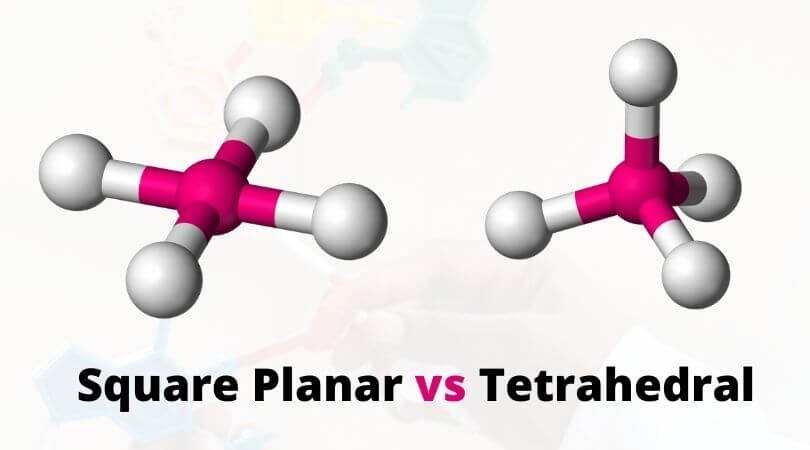
There are many molecular shapes that are formed due to the unique distribution of the atoms in the complexes. Still, many people struggle to understand the differences between square planar and tetrahedral geometry, as both these geometries have the same coordination number. There are striking differences between these molecular shapes, and understanding it better, let us first check out the arrangement of atoms in both of these molecular shapes.
Square Planar Geometry
The molecules that form square planar geometry have one central atom surrounded by the four constituent atoms in the corner of its square plane. This distribution of atoms in the central atom’s square plane is where it gets the name “square planar.” The bonds between the atoms in this geometry are 90 degrees. Mostly the transition metals that have the electron configuration ending in d8 form this type of molecular geometry.
Tetrahedral Geometry
People tend to get confused between both these molecular geometry because both square planar and tetrahedral has the coordination number of 4. The distribution of ligands in this geometry forms a structure similar to a pyramid, where ligands are located at every corner of the pyramid with one central atom in the middle of the structure. The bond angle between the ligands is higher than 90 degrees.
Now that we know how exactly the atoms are arranged in square planar and tetrahedral geometry let us go through the differences between these two geometries that can help you differentiate the shapes better.
Square planar vs Tetrahedral
| Square Planar | Tetrahedral |
| The square planar geometry has one central atom that is surrounded by the four constituent atoms. | One atom is located at the center of the four atoms, forming a structure similar to a tetrahedron. |
| The bond-angle between the atoms in the square planar geometry is 90 degrees. | The bond-angle between the ligands in the tetrahedral geometry is 109.5 degrees. |
| The coordination number of the complexes forming such molecular geometry is 4. | The complexes forming a tetrahedral geometry also have the coordination number of 4. |
| This type of geometry is mostly prevalent in the transition metal complexes. | The compounds having no lone pairs of electrons form a tetrahedral structure. |
| The square planar complexes form a four-tiered diagram in CFT. | Tetrahedral complexes form a two-tiered crystal field diagram. |
| The complexes forming square planar geometry has the electron configuration ending in d8. | The configuration of the electrons in tetrahedral complexes can be from d0 or d10. |
| Transitions metals such as Rh(I), Ir (II), etc has square planar geometry. | Methane or CH4 has the tetrahedral geometry where the Carbon atom is in the central position of the complex. |
| Square planar complexes are low spin as electrons tend to get paired instead of remaining unpaired. | Tetrahedral complexes are high spin because electrons in the complex tend to go the higher energy levels instead of pairing with other electrons. |
How does CFT help in understanding these geometries better?
The square planar complex forms a four-tiered crystal filed according to the CFT as four energy levels include dx2-y2, dxy, dz2, and ( dxz, dyz). The splitting of the crystal field into four energy levels helps in determining the distribution of the atoms in square planar geometry.
For tetrahedral complexes, the crystal field splitting diagram is the complete opposite of the octahedral diagram. A tetrahedral complex has the ligands in all the places where the octahedral complex doesn’t have. It has two-tiered crystal field diagrams corresponding to its two energy levels. The first set of orbitals are dxy, dxz and dyz, while another set has dx2-y2, dz2 orbitals. These two energy levels form the structure like a tetrahedron, and hence the ligands are distributed in that pattern.
The relation between Tetrahedral and square planar
Studying both these structures in detail helped us know that a tetrahedral geometry can be flattened to get a square planar geometry. And this conversion of the structure is a pathway for the isomerization of the compounds having tetrahedral shape.
Concluding Remarks
Both square planar and tetrahedral geometry can be differentiated with ease visually as well as looking at their CFT model. The same coordination number can confuse you, but keeping the points mentioned above in mind can easily help you recognize the geometries.