The chemical formula AsF5 represents Arsenic Pentafluoride. The compound exists as a colorless gas at room temperature and condenses into a yellow liquid at -53°C. The combination of Arsenic and Fluorine in the compound makes it extremely toxic. It hydrolyzes readily in water and has been restricted for use in aqueous systems.

Arsenic Pentafluoride is manufactured by the fluorination of elemental Arsenic. This reaction is shown below:
2As + 5F2 → 2AsF5
It can also be prepared by the reaction of Fluorine with Arsenic Trifluoride or Arsenic Oxides. Arsenic Pentafluoride is used as a doping agent in the manufacture of electrical polymers. The compound also forms halide complexes- hexafluoroarsenate is a notable example.
As with any Arsenic compound, it must be handled with care due to its high toxicity.
AsF5 has the following properties:
| Name of the molecule | Arsenic Pentafluoride (AsF5) |
| No. of valence electrons | 5 + (5 x 7) = 40 valence electrons |
| Hybridization of the central atom | sp3d |
| Bond Angles | 90°,120° |
| Molecular Geometry of AsF5 | Trigonal Bipyramidal Molecular Geometry |
Contents
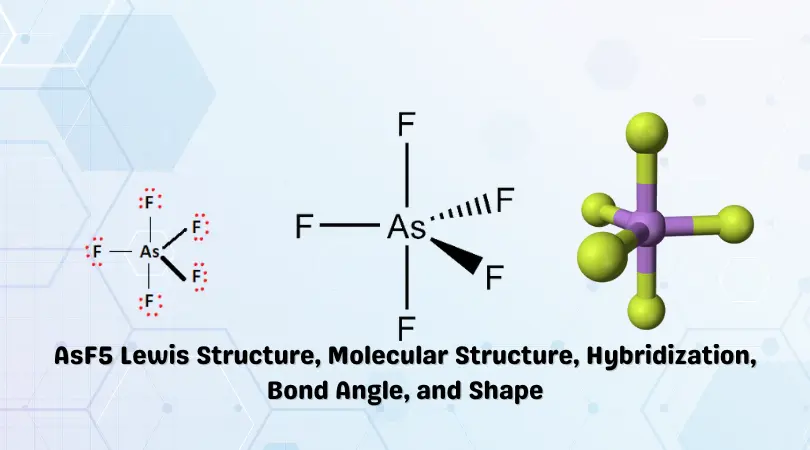
AsF5 Lewis Structure
A Lewis dot structure is a schematic representation of the arrangement of atoms, electrons, and chemical bonds within a particular molecule. The simple diagram uses dots, lines, and chemical symbols of elements to represent valence electrons, chemical bonds, and constituent atoms. It forms the basis for preliminary study and gives insight into molecular structure and chemical polarity.
The first step in obtaining a particular Lewis structure is determining the total number of valence electrons available.
Valence Electrons
Valence electrons are those electrons that lie in the outermost shell of the atom. Here, the force of attraction from the nucleus on these electrons is weak. Therefore, these electrons break free to participate in the bond formation or electron exchange.
Each atom in the molecule contributes a set number of valence electrons depending upon their atomic number and position on the periodic table. Valence electrons are the building blocks of our Lewis structure. AsF5 comprises Arsenic and Fluorine. Let us determine the number of valence electrons in AsF5.
Arsenic is in group 15 of the periodic table with the electronic configuration [Ar] 3d¹⁰4s²4p³. Therefore, the single Arsenic atom contributes 5 x 1 = 5 valence electrons.
Fluorine is a halogenic compound. It belongs to group 17 of the periodic table and has the electronic configuration [He] 2s22p5. Therefore, the five Fluorine atoms present contribute: 7 x 5 = 35 Valence Electrons.
Therefore, the total number of valence electrons in Arsenic Pentafluoride [AsF5] is given by:
5[As] + 35[F] = 40 valence electrons.
Lewis Structure Assembly
Now that the number of valence electrons has been determined, we can now begin to arrange them in our Lewis structure. Arsenic is the least electronegative element in this context and is placed at the center of the molecule. The five Fluorine atoms are placed around the central Arsenic atom as shown in the skeletal structure below.

40 valence electrons are available and we start by placing two electrons each between atoms to represent covalent bonds. The remaining electrons are placed around the atoms to fulfill the outer-shell requirements in accordance with the octet rule. The diagrams below offer a visual representation of the structure so far:


From the figures above, we can see that all 40 valence electrons have been used in the Lewis structure. The Fluorine atoms have complete outer shells with 8 valence electrons attached to each atom. However, a keen eye will notice that the central Arsenic atom has 10 valence electrons bonded to it- 5 of its own valence electrons and 5 additional electrons through covalent bonding with Fluorine. Arsenic is an exception to the octet rule in that it can have more than 8 outer-shell electrons. This makes the structure stable in spite of this unusual quirk.
We can use the concept of formal structures to verify the stability of AsF5
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| As | 5 | 0 | 10/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
Formal charges are zero for all the atoms in AsF5. This indicates stability in the arrangement. Therefore, the Lewis structure for Arsenic Pentafluoride is given below:

AsF5 Hybridization
The hybridization of a compound gives information about its energy levels, orbital structure, and the nature of the bonds. An easy way to determine the hybridization of an atom is to calculate the number of electron domains present near it. The bond between atoms (covalent bonds) and Lone pairs count as electron domains. Two electron domains correspond to an sp hybridization, three domains correspond to an sp2 hybridization, and so on.
AsF5 comprises five covalent bonds between the central Arsenic and Fluorine atoms. This corresponds to five electron domains being present around the central atom.
Therefore, the hybridization of Arsenic in AsF5 is sp3d.
AsF5 Bond Angles
The Fluorine atoms in Arsenic Pentafluoride repel each other in accordance with the VSEPR theory resulting in bond angles of 90° and 120°.
AsF5 Molecular Geometry and Shape
Some insight into the molecular geometry of AsF5 can be gained by observing the Lewis structure above. Three Fluorine atoms are in the same plane and two are near the equatorial region. Repulsion between regions results in the atoms being driven apart into a Trigonal Bipyramidal shape.

We can use the A-X-N method to confirm this.
‘A’ here represents the central Arsenic atom. Therefore, ‘A’ = 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, five Fluorine atoms are bonded to the central Arsenic atom.
Therefore, X =5.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 0 as there are no lone pairs attached to the Arsenic atom.
Therefore, that would give us an AX5 arrangement for Arsenic Pentafluoride.
From the A-X-N table below, we can determine the molecular geometry for AsF5.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX3N | Trigonal Pyramidal | 109.5 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
From the above table, it can be observed that an AX5 arrangement corresponds to a Trigonal Bipyramidal Molecular geometry.
Therefore, AsF5 has a Trigonal Bipyramidal molecular geometry and shape.
CONCLUDING REMARKS
Let’s quickly summarize the features of Arsenic Pentafluoride
- AsF5 is a highly toxic gas at room temperature. Lack of application due to handling difficulties and exposure risk.
- AsF5 comprises Arsenic as the central atom surrounded by five Fluorine atoms.
- In its most stable state, the central Arsenic atom forms five covalent bonds with the surrounding Fluorine atoms. Arsenic is an exception to the octet rule in that can have more than 8 electrons in its outermost shell.
- The hybridization of the central Arsenic atom in AsF5 is sp3d.
AsF5 has a Trigonal Bipyramidal molecular geometry and shape resulting in bond angles of 90° and 120°.