The chemical formula HNO2 represents Nitrous Acid. HNO2 is also known as Dioxonitric(III) acid.
It is a weak acid and exists only in specific conditions, i.e., in solution (cold and dilute), as a gas, or in the form of nitrite salts. It appears to be pale blue as a solution. Its existence in these peculiar states is due to the fact that HNO2 is unstable and decomposes rapidly in its free form.
Nitrous acid decomposes into Nitric Oxide (NO) and Nitric Acid (HNO3) at elevated temperatures. Again, due to its unstable nature, it can react as either an oxidizing or reducing agent. One of the more prominent Nitrites (salts of Nitrous acid) is NaNO2 (Sodium Nitrite). It is mainly used as a preservative in the processed meats industry.
Nitrous acid plays an important role in the production of diazonium salts. HNO2 reacts with amines to form diazonium salts, which play a vital role in the production of azo dyes. This simple synthesis is the crucial step in the production of commercial dyes.
Nitrous acid also finds use as a precursor in the production of Adipic acid, which is a key component in the production of Nylon. It also reacts with aliphatic alcohols to produce alkyl nitrites which act potently to counteract Vasoconstriction.
It has the following properties:
| Name of the molecule | Chlorine Trifluoride (HNO2) |
| No. of valence electrons | 1 + 5 + (6 x 2) = 18 valence electrons |
| Hybridization of the central atom | sp2 |
| Bond Angles | 120° |
| Molecular Geometry of HNO2 | Bent Molecular Geometry |
Contents
HNO2 Valence Electrons
Each atom in the molecule contributes valence electrons from their outermost shells. We can use these electrons to form the Lewis structure for Nitrous Acid.
Nitrous acid comprises two Oxygen atoms, one Hydrogen atom, and one nitrogen atom.
Nitrogen is in group 5 of the periodic table with the electronic configuration 1s22s22p3.Therefore, the lone Nitrogen atom contributes 5 x 1 = 5 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the single Hydrogen atom contributes 1 x 1 = 1 valence electron.
Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of -2. Oxygen’s electronic configuration is 1s22s22p4.
Therefore, the two Oxygen atoms present in the molecule contribute 6 x 2 = 12 valence electrons.
Thus, the total number of valence electrons in Nitrous Acid [HNO2] is given by:
1[H] + 5[N] + 12[O] = 18 valence electrons.
HNO2 Lewis Structure
The Lewis structure of a compound represents a graphic arrangement of constituent atoms present in a molecule mixture. It tells us about bond nature, molecular geometry and hybridization among other properties.
Lewis structures also help predict polarity and reactivity.
We have determined the valence electrons available to help form the structure in the previous section. The 18 valence electrons available will now be redistributed between the constituent elements present in HNO2 to form covalent bonds and fulfill the octet rule.
Whenever there’s a Hydrogen atom attached to a polyatomic ion, we can safely say that it is an acid. Here, Nitrogen will act as the central atom, and Hydrogen will be placed at the end, adjacent to one of the Oxygen atoms. This arrangement is shown in the skeleton structure below.

Two valence electrons are placed between each of the atoms in the compound, and covalent bonds are formed.


Now that we’ve formed covalent bonds, we can start fulfilling the octet requirements of the outermost atoms using the remaining valence electrons. Valence electrons are placed on the two Oxygen atoms first. Once that is taken care of, we place the remaining electrons on the nitrogen atom. We’ve used up all of our Valence electrons now. This arrangement of electrons is shown below.
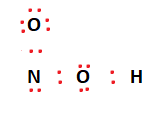
It can be observed that the octet conditions for Oxygen and Hydrogen are met. However, Nitrogen still needs two more electrons to fulfill the octet rule. This can be done by moving over two valence electrons from an adjacent Oxygen atom to form a double bond with the Nitrogen atom. This makes the structure more stable and fulfills the octet rule for all the constituent atoms as well.
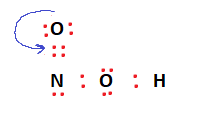
To verify the arrangement shown above, we can calculate the formal charges.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| H | 1 | 0 | 2/2 | 0 |
| N | 5 | 2 | 6/2 | 0 |
| O | 6 | 4 | 4/2 | 0 |
| O | 6 | 4 | 4/2 | 0 |
The formal charges being 0 for all of the atoms in the HNO2 molecule tells us that the electron arrangement shown above is stable.
Therefore, the Lewis Structure for the HNO2 is represented as follows:
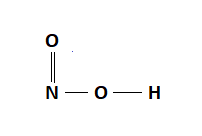
HNO2 Hybridization
Hybridization is a quantum phenomenon where energy is redistributed from atomic orbitals to form new hybrid orbitals that have equivalent energy. These new hybrid orbitals aid in bond formation and are responsible for molecular geometry and other molecular properties.
To determine the hybridization of Nitrous acid (HNO2), we first determine the number of electrons domains. Lone pairs and covalent bonds with other atoms contribute to being electron domains.
Two domains give us an sp hybridization. Three domains give us and sp2 hybridization and so on.
Therefore, in this case, there are three covalent bonds present. This gives us an sp2 hybridization state.
Nitrous Acid (HNO2) has a hybridization of sp2.
HNO2 Bond Angles
According to the VSEPR theory, the atoms will repel each other in the presence of lone pairs and electrons, giving rise to a bond angle of 120°.
HNO2 Molecular Geometry
To determine the molecular geometry of Nitrous acid, we must first observe its Lewis structure once more. There are three covalent bonds present, with the Hydrogen atom being on the outermost end. There is also a lone pair attached to the Nitrogen atom.
The electron clouds over these atoms will repel each other, pushing the Oxygen atoms downwards. This gives it a bent shape.
To verify this, we can use the A-X-N method.
‘A’ represents the central atom Nitrogen. Therefore, ‘A’ = 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, two Oxygen atoms are bonded to the central Nitrogen atom.
Therefore, X =2.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1 as there is one lone pair attached to the central Nitrogen atom.
Therefore, that would give us AX2N for the HNO2 molecule.
From the A-X-N table below, we can determine the molecular geometry for HNO2.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
From the above table, it can be observed that an AX2N arrangement corresponds to a Bent Molecular geometry.
Therefore, Nitrous acid (HNO2) has a bent molecular geometry.
HNO2 Polarity
To determine the polarity of the HNO2, we must first account for its properties. These include its electronegativity, its molecular geometry, and its resulting dipole moment if any.
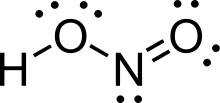
Let’s consider the molecular geometry of HNO2 already discussed above. There are two Oxygen atoms bonded to the central Nitrogen atom. There is also a Hydrogen atom present. This OH group resides on one end of the molecule, giving it its bent shape.
Therefore, the HNO2 molecule is asymmetric. This leads to an unequal distribution of valence electrons.
In this case, the Hydrogen end is more electropositive while the Oxygen end is more electronegative. This difference in electronegativity is responsible for the poles shown in the figure below.

Therefore, due to its asymmetrical shape, there is an unequal sharing of valence electrons. This, combined with the significant difference between the two ends of the molecule represented above, proves that Nitrous acid (HNO2) is a polar molecule.
Concluding Remarks
Let’s quickly summarize the salient features of HNO2
- HNO2 comprises a central Nitrogen atom bonded to Oxygen atoms and Hydrogen.
- In its most stable state, one of the Oxygen atoms forms a double bond with the Nitrogen atom to fulfill the octet rule. The Hydrogen atom placed at one end is bonded to an adjacent Oxygen atom.
- The hybridization of Nitrous acid is sp2.
- HNO2 has a Bent molecular structure with bond angles of 120°.
- HNO2 is a polar molecule due it asymmetric geometry and significant poles at each end.




