PH3 molecule, also known as phosphine, is a colourless, flammable and toxic gas compound. It is a respiratory poison and is highly dangerous to life at 50 ppm. It is a constituent of Earth’s atmosphere at significantly low and highly variable concentrations. Phosphine is also a general name given to the class of organophosphorus compounds of substituted phosphanes.
| Name of molecule | Phosphine ( PH3) |
| No of Valence Electrons in the molecule | 8 |
| Hybridization of PH3 | NA |
| Bond Angles | 93.5° |
| Molecular Geometry of PH3 | Trigonal pyramidal |
We shall now learn about the Lewis structure of this molecule to gain a better understanding of its physical and chemical properties. Let’s do it! To determine its Lewis Structure, we will first calculate the total number of valence electrons of the molecule, as it will help us comprehend the Lewis structure.
Contents
PH3 valence electrons
Valence electrons are the electrons present in the outermost shell of the atom. These electrons are the ones that participate in bond formation. To determine the Lewis Structure of any given molecule, it is crucial to know the total number of valence electrons for the molecule. Here we will find out the total number of valence electrons for PH3 by looking at the valence electrons of atoms individually first and then adding it.
Phosphorus has five valence electrons in its outer shell.
Hydrogen has one valence electron in its outer shell. But there are three hydrogen atoms in this molecule, due to which we will multiply the number by 3.
Hence, now there are three valence electrons for all hydrogen atoms in total.
= 5 + 3
= 8
There are eight valence electrons for the Phosphine or PH3 molecule.
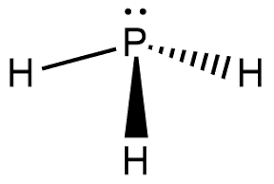
PH3 Lewis Structure
Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement of other atoms.
Here for PH3, the phosphorus atom will take the central position as Hydrogen atoms cannot take a central position in the Lewis Structure. So we will place the Phosphorus atom in the centre, and all the other Hydrogen atoms will be placed surrounding it.
Once you have done that, place a pair of electrons between each Hydrogen and Phosphorus atom. Doing this, we will use up to six valence electrons out of 8. This will also result in the complete outer shell for the Hydrogen atom, as it only needs two valence electrons to have a stable structure.
Two electrons that do not participate in the bond formation are placed on the central atom as lone pair or nonbonding pair of electrons.
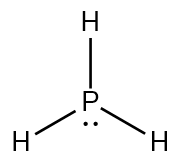
Hence, this is the Lewis Structure of the PH3 molecule where there are three bonding pairs of electrons and a lone pair of electrons on the central atom.
PH3 Hybridization
This may come as a surprise to some, but there is no defined hybridization for the Phosphine molecule. If you are aware of the Drago Rule, then you must know that we don’t need to consider the molecule’s hybridization if:
- The central atom has at least one lone pair of electrons.
- The electronegativity of the central atom is 2.5 or less.
- The central atom belongs to the group 13, 14, 15 or 16 and is from periods 3 to period 7.
- The number of sigma bonds and lone pairs is 4.
Here in PH3, the electronegativity value for Phosphorus is 2.19 ( less than 2.5), and it also has one lone pair of electrons. Apart from this, it also belongs to group 15. As a result, there is no hybridization for the PH3 molecule. Because it is a Drago molecule, there is no hybridization; instead, pure p-orbitals participate in bond formation.
PH3 Bond Angles
Although Phosphine or PH3 molecule resemble NH3 molecule, there is a difference in their bond angles. The central atom forms three sigma bonds with Hydrogen atoms and has a lone pair of electrons. The bond angles are approximately 93.5° for the PH3 molecule.
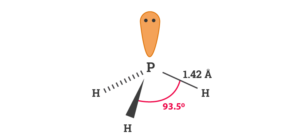

PH3 Molecular Geometry and Shape
In this molecule, Phosphorus has one lone pair of electrons along with three bonding pairs of electrons. According to the VSEPR theory, there are repulsive forces between bonding pairs of electrons, bonding and nonbonding pairs of electrons, and even nonbonding electrons.
As a result, this lone pair tries to remain as far as possible from these bonding pairs of electrons. The molecule attains a trigonal pyramidal geometry to minimize these repulsive forces. All the bonding pairs of electrons are at the base of the pyramid of geometry, and the lone pair is at the top.
Hence the molecular geometry for the PH3 molecule is a trigonal pyramidal.
Concluding Remarks
To summarize this blog post on the PH3 molecule, we can conclude the following:
- A Phosphine molecule comprises one Phosphorus atom and three Hydrogen atoms.
- There are eight valence electrons for the PH3 molecule.
- Phosphorus atom is in the centre forming single bonds with three Hydrogen atoms and also has a lone pair of electrons in its Lewis Structure.
- The bond angle for the PH3 molecule is 93.5°.
- The molecular geometry and shape of the PH3 molecule is a Trigonal pyramid.