The chemical formula AsF3 represents Arsenic Trifluoride. The compound exists as a colorless liquid at room temperature and decomposes in water. It is also soluble in alcohols, ethers, and ammonia solutions.
Arsenic Trifluoride is formed when Hydrogen Fluoride reacts with Arsenic Trioxide. This reaction is shown below:
6HF + As2O3 → 2AsF3 + 3H2O
Arsenic Trioxide also reacts with Sulfuric acid and Calcium Fluoride to give AsF3.
It can be used as a fluorinating agent to convert non-metallic chlorides to fluoride-based compounds. It is also used as a catalyst, an ion implantation source, and a dopant.
AsF3 has the following properties:
| Name of the molecule | Arsenic Trifluoride (AsF3) |
| No. of valence electrons | 5 + (3 x 7) = 26 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 96° |
| Molecular Geometry of AsF3 | Trigonal Pyramidal Molecular Geometry |
Contents
AsF3 Valence Electrons
Valence electrons are used in Lewis Structures to represent atomic bonds between atoms and electrons possessed by the individual atoms.
These valence electrons reside in the outermost shell of an atom, where the force of attraction from the nucleus is weak. This allows the valence electrons to break free from the atom to take part in chemical reactions.
Using the number of valence electrons available to us, we can determine the Lewis structure for a given compound.
Let us calculate the number of Valence electrons in AsF3.
Arsenic Trifluoride comprises three Fluorine atoms and one Arsenic atom.
Arsenic is in group 5 of the periodic table with the electronic configuration [Ar] 3d¹⁰4s²4p³. Therefore, the single Arsenic atom contributes 5 x 1 = 5 valence electrons.
Fluorine is a halogenic compound. It belongs to group 17 of the periodic table and has the electronic configuration [He] 2s22p5.
Therefore, the three Fluorine atoms present contribute: 7 x 3 = 21 Valence Electrons.
Therefore, the total number of valence electrons in Arsenic Trifluoride [AsF3] is given by:
5[As] + 21[F] = 21 valence electrons.
AsF3 Lewis Structure
As you already know, the Lewis structure of a molecule or compound helps give insight into various molecular properties. Lewis structures are stable schematic representations of the arrangement of atoms in a given molecule.
Lewis structures use the valence electrons contributed by the constituent atoms to show the bond formation and octet fulfillment.
We already know the number of valence electrons available to us. This has been calculated in the previous section to be 26.
Next, we arrange Arsenic and Fluorine atoms in a sort of skeletal arrangement. Arsenic takes its place as the central atom and facilitates bond formation. Covalent bonds between Arsenic and Fluorine are formed by making use of the available valence electrons. This is represented in the figure below.

The outermost shell requirements of atoms are addressed in accordance with the octet rule. Valence electrons are used to fulfill the outermost shells of the surrounding Fluorine atom.
Finally, this leaves us with two valence electrons. This will act as a lone pair and will be attached to the central Arsenic atom to fulfill its octet requirements.
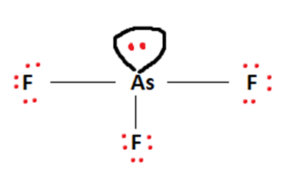
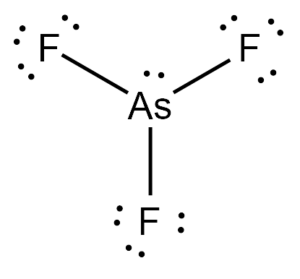
With this, our Lewis Structure is complete. We can verify its stability by calculating the formal charges of each constituent atom in the AsF3 molecule.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| As | 5 | 2 | 6/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
The formal charges being 0 for all of the atoms in the AsF3 molecule tells us that the Lewis structure obtained above is stable.
AsF3 Hybridization
The hybridization of a compound gives information about its energy levels, orbital structure, and the nature of the bonds.
AsF3 comprises three covalent bonds between the central Arsenic and Fluorine atoms. In addition to this, there is also a lone pair on the central Arsenic atom. This means that there are four electron domains.
Therefore, the hybridization of Arsenic in AsF3 is sp3.
AsF3 Bond Angles
VSEPR theory dictates that like electron pairs repel each other. As such, the Fluorine atoms repel each other accordingly. This gives AsF3 bond angles of approximately 96°.
AsF3 Molecular Geometry and Shape
As we discussed earlier, the Lewis structure of a compound helps determine its various properties. Molecular geometry is one of those properties, and we must understand the elemental arrangement from the Lewis structure to proceed.
The Lewis structure observed tells us that the Arsenic has a steric number of 4 with three covalent bonds and one lone pair attached. This gives it a trigonal pyramidal molecular geometry.

We can use the A-X-N method to confirm this.
‘A’ here represents the central Arsenic atom. Therefore, ‘A’ = 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, three Fluorine atoms are bonded to the central Arsenic atom.
Therefore, X =3.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1 as there is one lone pair attached to the Arsenic atom.
Therefore, that would give us an AX3N arrangement for Arsenic Trifluoride.
From the A-X-N table below, we can determine the molecular geometry for AsF3.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX3N | Trigonal Pyramidal | 109.5 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
From the above table, it can be observed that an AX3N arrangement corresponds to a Trigonal Pyramidal Molecular geometry.
Therefore, AsF3 has a trigonal pyramidal molecular geometry. The lone pair and a steric number of 4 also help us conclude that Arsenic Trifluoride has a Tetrahedral electronic shape.
Concluding Remarks
Let’s quickly summarize the salient features of Arsenic Trifluoride
- AsF3 comprises an Arsenic atom surrounded by three Fluorine atoms. There’s also a lone pair of electrons attached to the central Arsenic atom.
- In its most stable state, the central Arsenic atom forms three covalent bonds with the surrounding Fluorine atoms.
- The hybridization of the central Arsenic atom in AsF3 is sp3.
- AsF3 has a Trigonal Pyramidal molecular geometry and a Tetrahedral electronic shape with bond angles of approximately 96°.