Chemistry is the one subject that needs a lot of understanding of the terms such as an atom, molecules, electrons, formula units, etc. The basics of chemistry involve the knowledge of all these terms, to make it easy for all the people who are interested in chemistry we are going to distinguish between the words such as molecules and formula unit in this article of Formula unit vs Molecule
Formula Unit vs Molecule
To start with the basics, an atom is a word we use to define the tiniest particle in chemistry. An atom has electrons, protons, and neutrons. Like an atom of oxygen has eight electrons. When we talk about atoms that have reacted with each other the end product is a molecule. So primarily a molecule is made up of atoms sometimes different types of atoms other times same kinds of atoms. The atoms that react together share a bond, and there are many types of bonds such as a covalent bond, ionic bond, etc.
There are two atoms of Oxygen that form one molecule of Oxygen. Similarly, two atoms of Hydrogen and one atom of oxygen forms a molecule of H2O which is commonly known as water. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Molecules are the smallest part of any given compound. This molecule may or may not consist of same types of atoms. Molecules are generally discussed in the chemical reactions for finding the molecular weight, moles, etc. in this reaction.
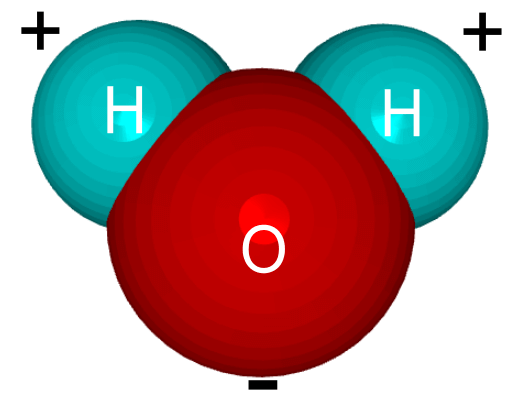
It is essential to know what a molecule is made up of to know the other terms in chemistry. It is also helpful in balancing the equations, doing calculations and finding the molecular formula of the compound whereas the term formula unit is entirely different from the term molecule.
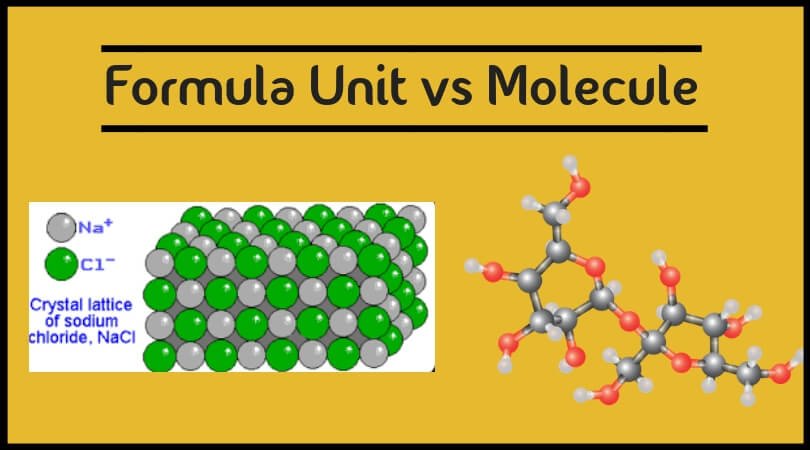
A formula unit is a term used for defining the whole lesser number of ratios of ions in an ionic compound. An ionic compound is made up of a metallic and non-metallic ion. When we look at any given ionic compound, it is made up of molecules, out of which one molecule is positively charged, and the other one is negatively charged. The positively charged molecules are known as a positive ion, and the negatively charged molecule is known as a negative ion. There is a ratio between these positive and negative ions of which the lowest number is taken to define the difference. This whole lowest number is made to determine the ratio of ions is called the formula unit.
Formula unit helps us in giving an empirical formula that can be used for understanding the compound. It is the unit that represents an entire compound. For example, we consider NaCl compound here Na or sodium is a positive ion and Cl or chlorine is a negative ion. These positive and negative ions will be arranged in a crystal lattice in the compound to give the final ionic compound. A formula unit is helpful in solving stoichiometric calculations. The formula unit is taken as a single entity in the calculation.
To sum it up and conclude this article Formula Unit vs Molecule, a molecule is made up of atoms that are covalently bound to each other, and it is essential to know the molecule and its atoms for knowing their formula unit, molecular weight, moles, etc. A formula unit is the lowest possible number used to define the ratios of ions in ionic as well as covalent compounds.




“one atom of Hydrogen and two atoms of oxygen forms a molecule of H2O which is commonly known as water.”
Hello !
two atoms of hydrogen and one atom of oxygen . it was mentioned reversely.
please correct it.
Thank you for suggesting a correction. I have done the edit. Thank you for reading.