Methylene chloride, also known as Dichloromethane (DCM), is an organic chemical compound. CH2Cl2 is the chemical formula for DCM. It is a colorless and volatile liquid with a sweet smell. The compound is naturally derived from the volcanoes, wetlands and other oceanic sources. It has many uses, but majorly it is used in the food industry. In this article, we will know the structure, molecular geometry, applications and other chemical properties in detail.
Contents
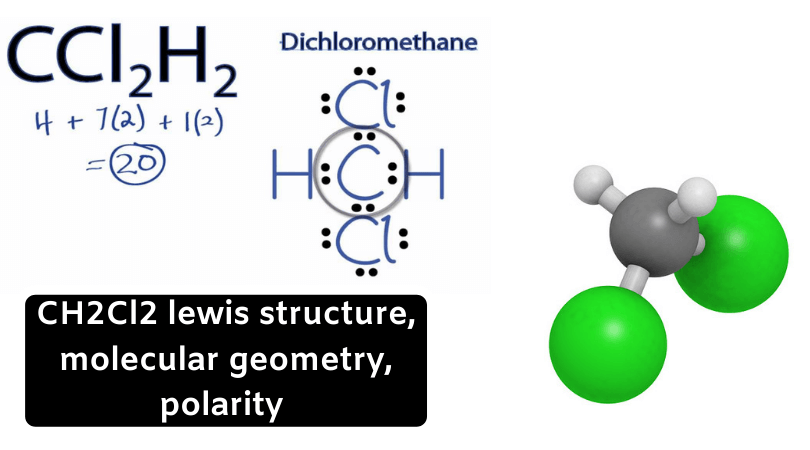
CH2Cl2 Lewis structure
For understanding the properties and structure of any chemical compounds, including organic ones, its lewis structure is of the utmost importance. Lewis structure is a theory that helps in understanding the structure of a given compound, based on the octet rule. According to the octet rule, a molecule should have eight electrons in its outer shell to become inert or stable. For this compound, there is one molecule of Carbon, two molecules of Hydrogen and two molecules of Chlorine.
To know the lewis structure, it is vital to find the number of valence electrons in the compound. Valence electrons are the sum total of the electrons every molecule has in their outer shell in a compound. These electrons include the ones that participate in bond formation as well as the ones that don’t participate in forming bonds. The electrons that are involved in bond formation are called bonding pairs of electrons. Whereas the ones that do not participate in forming any bonds are called lone pairs of electrons or non-bonding pairs of electrons.
In Lewis structure the lines represent the bonds and dots represent the valence electrons. When we talk about CH2Cl2, Carbon is less electronegative than Chlorine atoms. To understand the Lewis structure lets first calculate the total number of valence electrons for Dichloromethane.
Carbon has four valence electrons, Hydrogen has one valence electrons and like all halogens, Chlorine has seven valence electrons.
Total number of Valence electrons = 4 + 2*1 + 2*7
= 4+2+14
= 20
There are twenty valence electrons in the compound, and four bonds are formed. Central carbon atom forms two bonds with both Hydrogen and Chlorine atoms. Thus four valence electrons of Carbon, two electrons of Hydrogen and Chlorine each participate in the bond formation.
Hybridization of Dichloromethane

When two or molecules participate in the bond formation, their orbitals overlap due to the sharing of electrons. These overlapped orbitals are called hybrid orbitals. The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3.
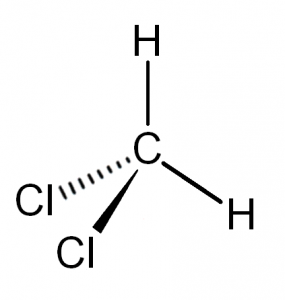
Molecular Geometry of Dichloromethane
It is comparatively easy to understand the molecular geometry of a compound after knowing its Lewis structure and hybridization. The arrangement of the molecules in this compound is such that the Carbon atom is in the central atom, one Hydrogen atom is on the upper topmost position and the other one is on the left side of the central atom. Similarly, one chlorine atom is to the right of Carbon and the other one is one the downward position of the central atom. As the hybridization is sp3, the molecular geometry of Dichloromethane becomes tetrahedral. The shape of the compound is a trigonal pyramidal.
Polarity of Dichloromethane
The polarity of any compound depends on the lone pairs of electrons and symmetry of the compound. It also depends on the electronegativity of the molecules participating in the formation of the compound. Here Hydrogen atom is less electronegative than chlorine atom and hence, there is a net dipole moment in the compound. Also, the arrangement of the bonded pairs is asymmetric, which makes Dichloromethane polar.
Physical Properties
Now that we know all about the chemical properties and structures of CH2Cl2 let’s have a look at its physical properties.
| Property of the compound | Experimental values |
| Density of DCM | 1.3226 g/cm3 |
| Molecular weight of DCM | 84.93 g/mol |
| Boiling point of DCM | 39.60C |
| Melting point of DCM | -97.60C |
Uses of Dichloromethane
- DCM is used as a solvent in the food industry and as a paint remover.
- It is also used as a degreasing agent.
- The compound is also used in the production of aerosol formulations.
Hazards of using Dichloromethane
- As the compound is highly volatile in nature, it can cause acute inhalation hazards. Prolonged exposure to DCM can cause dizziness, fatigue, headache and much more as a result of acute absorption of the gas.
- DCM is metabolized as Carbon monoxide in the body that can lead to Carbon Monoxide Poisoning in the body.
- It has also been linked to various types of cancer and thus is a carcinogenic compound.
- The compound is also not safe for people with heart-related issues as it can cause abnormal heart rhythms and heart attacks when inhaled for an extended period.
- In some cases, it can also irritate the nose and throat.
Concluding Remarks
I hope this article gives you detailed information about Dichloromethane. The compound has twenty valence electrons, out of which eight electrons participate in bond formation. It has sp3 hybridization and polar. DCM has tetrahedral molecular geometry and it is trigonal pyramidal in shape.