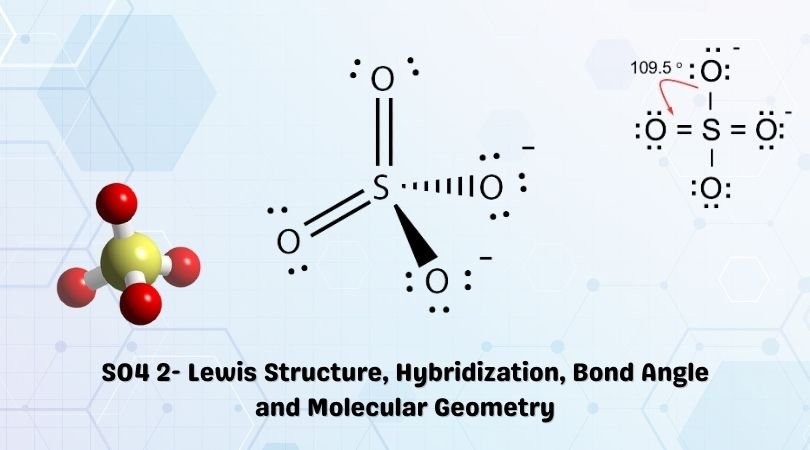
SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more.
| Name of molecule | Sulfate ion |
| No of Valence Electrons in the molecule | 32 |
| Hybridization of SO42- | sp3 hybridization |
| Bond Angles | Approximately 109.5° |
| Molecular Geometry of SO42- | Tetrahedral |
In this blog post, we will go through all the details related to this molecule. Right from valence electrons to shape, you will find everything related to SO42- ion here.
Contents
SO42- Valence electrons
For determining the Lewis Structure for any molecule, we first need to know the total number of valence electrons. These electrons are the ones that are present in the outermost shell of the atom and participate in forming bonds.
Here we have one Sulphur atom and 4 Oxygen atoms, and both these elements belong to the same group and have six valence electrons in their outermost shell.
But as there is a -2 charge on this molecule, it means that it accepts two additional electrons to attain a stable structure, and hence we have to consider these electrons as well.
Total valence electrons for SO42- = 6 + 6(4)+2 = 32
Hence there are a total of 32 valence electrons for the Sulfate ion.
SO42- Lewis Structure
The Lewis Structure of any molecule helps to understand the bonding of atoms in the structure. Apart from that, it also helps to know the molecular shape, polarity, and other properties of the molecule. Generally, while forming bonds with other atoms, every atom tries to follow an octet rule that states that an atom must have eight valence electrons to attain a stable structure. There are some exceptions to this rule certainly, but the majority of the atoms follow this rule.
For determining the Lewis Structure for SO42- molecule, we need to know the feeling:
- Total valence electrons
- The central atom in the molecule
- Arrangement of the atoms in the molecule
- Bonds formed in the molecule
Here as we already have the total number of valence electrons, we can start by determining the central atom. To determine which atom takes the central atom, always remember that the less electronegative atom takes the central position, and more electronegative atoms are arranged around it. This doesn’t apply to Hydrogen atoms, as these atoms never take the central position.
In SO42-, if you compare the electronegativities of Sulphur and Oxygen atoms, Oxygen is more electronegative than Sulphur atoms. So as a result of this Sulphur atom will take the central position, and all the Oxygen atoms are arranged around it.
So place a Sulfur atom in the center and Oxygen atoms around it. Now that you have the arrangement of the atoms in the molecule, we can look at the bonding of atoms.
As mentioned above, each atom will try to have eight valence electrons in its outer shell to attain a stable structure. So place a pair of electrons between each Oxygen and Sulphur atom to show a single bond.
Placing four single bonds will use eight valence electrons out of 32, which means we are only left with 24 valence electrons now. Start putting these electrons around the Oxygen atom, as Sulphur has shared four electrons with the Oxygen atoms.
Now doing this, you might wonder that this is the correct way to present the Lewis Structure, but it is suggested to calculate the formal charges to do so. You can either use our Formal charge calculator to find the charges on the atoms or use the following formula:
F.C = Valence electrons – Nonbonding pairs of electrons * ½ bonding pairs of electrons
Using this formula, you will find out that when there are four single bonds in this molecule, each Oxygen atom has a charge of -1, and the Sulphur atom has a charge of -2. So if you add up the charges, it is 4(-1)+ (-2) = -6, which is technically wrong as we have a charge of -2 on this ion.
Besides, the Sulphur atom cannot have a higher negative charge than the Oxygen atom as it is less electronegative than it. So this is not the correct Lewis Structure.
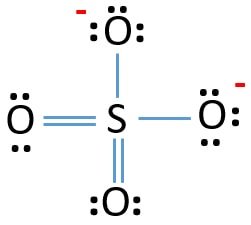
However, if you shift electrons from the Oxygen atom and form a double bond with the Sulfur atom, it might solve our problem. Repeat the same for another Oxygen atom as well. Now you have two double bonds and two single bonds in the structure.
Sulphur can accommodate more than eight valence electrons in its outer shell; it is okay if it exceeds the limit of 8. So always remember that elements like Phosphorus, sulfur, etc can have expanded octets to accommodate more electrons.
Calculating the formal charges for this Lewis Structure will give you the following values:
Sulfur has a net charge of zero.
Oxygen forming double bonds have a net charge of zero.
And the Oxygen atoms forming single bonds have a -1 charge; in total, there are two such atoms, and hence the ion has an overall charge of -2.
As these charges are now in accordance with the charges mentioned on the ion, this is the accepted Lewis Structure for the sulfate ion. Also, remember always to put brackets and mention the charge while writing the Lewis Structure for the ions.
Hence, this is the Lewis Structure for SO42-.
SO42- Hybridization
There are several ways and techniques to find out the Hybridization for any given atom in the molecule. But in my opinion, the easiest way to do this is by finding out the steric number or counting the number of electron regions around the central atom.
Here, sulfur forms bonds with four oxygen atoms, which means four electron regions are around it. To accommodate the electrons shared in these bonds, it needs to form 4 hybrid orbitals. As a result, there is a formation of one s-hybrid orbital and three p-hybrid orbitals. (Each s orbital can accommodate 2 electrons, and p orbital can accommodate 6 electrons). Hence SO42- ion has an sp3 hybridization.
SO42- Molecular Geometry
We can determine the molecular geometry of any given molecule using the VSEPR theory model and the AXN notation method. For example, for the Sulphate ion, the AXN notation would be AX4, as it forms bonds with four oxygen atoms. And as a result of this, it has a tetrahedral molecular geometry.
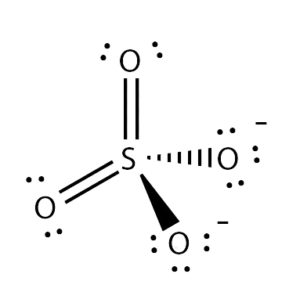
Therefore, SO42- ion has a tetrahedral molecular geometry.
SO42- Bond angles
The bond angles for molecules having a tetrahedral geometry are generally 109.5 degrees, but as there are double bonds, it might be close to this angle but not precisely 109.5 degrees.

SO42- Shape
As predicted by the VSEPR theory, a molecule that consists of the central atom forming bonds with four atoms has a tetrahedral shape; a sulfate ion also has a tetrahedral shape.
Concluding Remarks
To conclude this blog post on SO42- ion we can summarize the following from the information stated above:
- SO42- ion has one Sulfur atom, which takes the central position.
- All the Oxygen atoms are arranged around the central atom.
- Two out of four Oxygen atoms form double bonds with Sulfur atoms.
- There are a total of 32 valence electrons for this ion.
- As all the electrons are used up in bond formation, there are no lone pairs of electrons on the sulfur atom.
- The bond angles are approximately 109.5 degrees.
- It has a tetrahedral molecular shape and -2 charge as it accepts two additional electrons to attain a stable structure.
- The -2 charges are due to the two Oxygen atoms forming a single bond with the Sulfur atom.