The chemical formula SeO2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements.
SeO2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid.
SeO2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal.
The compound has the following properties:
| Name of the molecule | Selenium Dioxide (SeO2) |
| No. of valence electrons | 5+6+7 = 18 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 120° |
| Molecular Geometry of ClO3– | Bent Molecular Geometry |
This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape.
Contents
SeO2 Valence Electrons
SeO2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule.
Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s23d104p4.
Therefore, a single atom of Selenium contributes 1×6 = 6 valence electrons.
Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of -2. Oxygen’s electronic configuration is 1s22s22p4.
Therefore, two Oxygen atoms contribute 6 x 2 = 12 valence electrons.
Thus, the total number of valence electrons in Selenium Dioxide [SeO2] is given by:
6[Se] + 12[O] = 18 valence electrons.
SeO2 Lewis Structure
Selenium is the least electronegative and will act as the central atom. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure.
The valence electrons are first placed between the Selenium and Oxygen atoms to form covalent bonds.
The remaining valence electrons are placed on the outermost or most electronegative atoms first. In this case, that would be Oxygen. The two oxygen atoms fulfill their electron requirements in accordance with the octet rule.
The remaining two valence electrons go to Selenium and act as a lone pair.
As shown above, the central Se does not have an octet as it has only six valence electrons. This tells us that we need to move one of the outer lone pairs of valence electrons inward.
To verify if the above structure is stable, we need to calculate the formal charges on each of the atoms.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case, the formal charges are given by:
| Element | V | N | B/2 | FC |
| O | 6 | 6 | 2/2 | -1 |
| S | 6 | 2 | 6/2 | +1 |
| O | 6 | 4 | 4/2 | 0 |
The presence of positive and negative formal charges tells us that this may not be the most stable structure for SeO2. The presence of a positive +1 charge indicates the need for another double bond.
The structure is then modified as shown below:
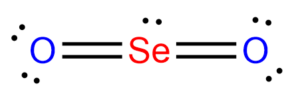
Upon calculating the formal charges, we see that they come up to zero. This tells us that the SeO2 compound forms two double bonds with Oxygen to obtain its most stable structure.
| Element | V | N | B/2 | FC |
| O | 6 | 4 | 4/2 | 0 |
| S | 6 | 2 | 8/2 | 0 |
| O | 6 | 4 | 4/2 | 0 |
Therefore, the Lewis structure of SeO2 is given below as:
SeO2 Hybridization
An easy method to determine the hybridization of an atom in an element is to observe the number of its electron regions or electron domains. Lone pairs and covalent bonds with other atoms contribute to being electron domains.
Two domains give us an sp hybridization. Three domains give us and sp2 hybridization and so on.
In this case, Selenium forms two double bonds with Oxygen and has a lone pair. This gives us three domains. However, the unique feature of SeO2 is that it exists as a polymer chain. This gives rise to another oxygen bond and gives us four domains.
The central atom, Selenium, then has a hybridization of sp3.
SeO2 Bond Angles
According to the VSEPR theory, the covalent between the oxygen atom and Selenium’s lone pair repel each other. This gives SeO2 a bond angle of 120°.
SeO2 Molecular Geometry and Shape
Selenium dioxide exists as a one-dimensional polymer and the central atom, Selenium, bears the connecting Oxygen atom.
For academic purposes, we can determine the molecular geometry and shape for a single component of the polymer chain. Using the steric method, we determine that with a steric number of 4, SeO2 has a “bent” molecular geometry or shape.
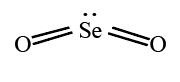
However, we must understand that SeO2 does not exist as a single molecule in nature. It occurs as a polymer chain with pyramidal geometry with Se-O bond lengths of 179 pm and terminal Oxygen bond lengths of 162 pm.
Concluding Remarks
Let’s quickly summarize the salient features of SeO2
- SeO2 consists of one Selenium atom bonded to two Oxygen atoms.
- In its most stable state, Selenium acts as the central atom and forms double bonds with the surrounding Oxygen atoms. In nature, however, the bonds are different.
- Due to its polymeric nature, the hybridization of SeO2 is sp3.
- SeO2 has a bent molecular structure with bond angles of 120°.




