The cyanate ion is an anion composed of one oxygen atom, one carbon atom, and one nitrogen atom, in the order [OCN]. It possesses 1 unit of a negative charge, borne by the nitrogen atom. It is an ambident nucleophile in nucleophilic substitution. The reason is that it can react to form an alkyl cyanate R-OCN (exception) or an alkyl isocyanate R-NCO (rule). It is relatively non-toxic in comparison with cyanides.
We will now learn about the Lewis structure of this molecule to understand its physical and chemical properties. The structure will help better understand the arrangement of atoms, bond formation and shape of the molecule.
| Name of molecule | Cyanate Ion |
| No of Valence Electrons in the molecule | 16 |
| Hybridization of NCO- | sp hybridization |
| Bond Angles | 180° |
| Molecular Geometry of NCO- | Linear |
We will first calculate the total number of valence electrons of the molecule, which will help comprehend the molecule’s Lewis structure. Let’s go!
Contents
NCO- Valence electrons
To know the Lewis structure of any given molecule, we first need to know the total number of valence electrons. These electrons present in the outermost shell of the electrons and participate in the bond formation with the neighbouring atoms. As we have one atom of Nitrogen, Carbon and Hydrogen each, we will first look at the valence electrons for these individual atoms.
As Nitrogen is a group 5 element, it has five valence electrons in its outer shell.
Carbon has four valence electrons present in its outer shell.
Oxygen has six valence electrons in its outer shell. But Oxygen also carries a negative charge.
Make sure to count every electron in the structure to get the right Lewis Structure for any molecule. And as there is an additional electron over here, we will count that too while calculating the total number of valence electrons for this ion.
Hence, 5+4+6+1 = 16
Therefore, the total number of valence electrons in the NCO- molecule is 16.
NCO- Lewis structure

To determine the Lewis Structure for any molecule, we need to know the following:
- Total number of valence electrons
- The central atom
- Arrangement of atoms around the central atom
Here as we already know that there are 16 valence electrons, we can determine the central atom for this ion.
The central atom for Cyanate will be the C atom since it is the least electronegative. Show a single bond between the central atom is Carbon, with its neighbouring atoms ( Oxygen and Nitrogen).
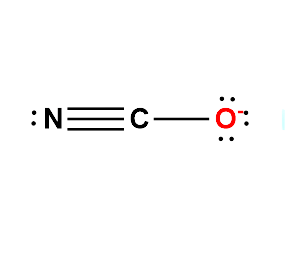
A double bond is added between C and O in the structure, and a second double bond is added between C and N. Alternatively, one triple bond is added either between C and O or between C, and N. Unshared electron pairs are added so that there is an octet of electrons around each atom. Delocalization electron pairs draw all the other equivalent resonance structures.
Therefore, the Lewis structures for NCO- are as follows:
The above Lewis structures indicate that the cyanate ion is capable of bonding at either the Nitrogen or Oxygen to a metal atom. However for Cyanate ion, the most accepted Lewis Structure if of that in which Carbon forms a triple bond with Nitrogen atom and a single bond with Oxygen atom.
NCO- Hybridization
One of the easiest and simple ways to find out the Hybridization of any molecule is to look at the electron domain regions around the atom. Here as Carbon is forming bonds with Nitrogen and Oxygen atoms, we will look at its Hybridization. Cabron forms a triple bond with the Nitrogen atom, which corresponds to one electron region, and it also forms a single bond with Oxygen, this is the second electron region. And as there are two electron regions for Carbon, it forms two hybrid orbitals to form bonds with these two atoms.
Carbon forms one s-hybrid orbital and another p-hybrid orbital to form a triple bond and a single bond with the neighbouring atoms. As a result, the Hybridization for the Carbon atom in NCO- ion is sp.
NCO- Molecular geometry
As we already know, O is the most electronegative; hence it should have the -1 formal charge, that there is a triple bond between N and C and a single bond exists between C and O. Hence there is no lone pair around the C atom. Looking at the arrangement of atoms and their valence electrons, it can be seen that the arrangement is planar, and as there are no lone pairs, there is no bent in its shape or geometry. From VSEPR theory, we know that this molecule would then be linear.
NCO- Bond angles
The bond angle for this ion is 180 degrees as it has a linear geometry, and all the atoms are arranged in the same plane.
NCO- Shape
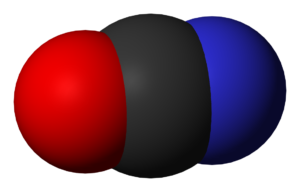
The shape of the Cyanate ion is linear.
Concluding Remarks
To conclude everything above stated in the blog post, we can say the following:
- NCO- ion has a negative charge as it accepts an additional electron to attain this structure.
- There are a total of 16 valence electrons for this ion.
- Carbon forms a triple bond with the Nitrogen atom and a single bond with the Oxygen atom.
- It has sp hybridization with bond angles of 180 degrees.
- As all the atoms are arranged in the same plane, it is a linear molecule.