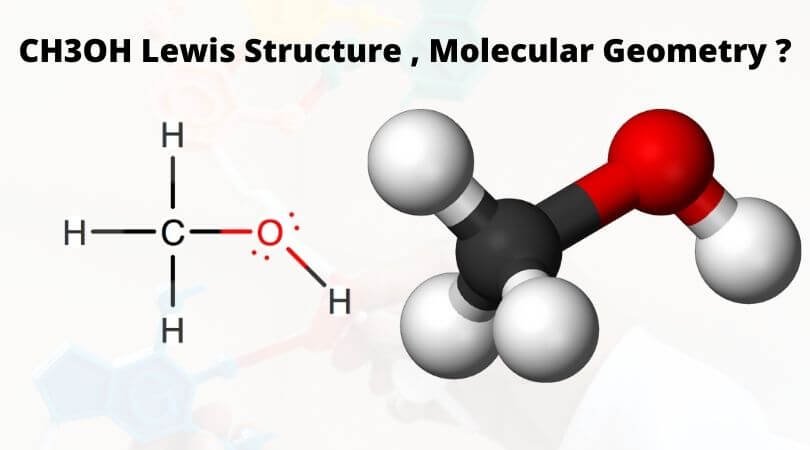
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. This compound has a hydroxyl group ( OH) attached to the methyl group, and that is where it gets its name of “methyl alcohol.” To make it easier for you to understand, assume that one hydrogen atom of methane or CH4 is substituted by a hydroxyl group, resulting in Methanol having the chemical formula of CH3OH.
Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol. The molecule’s structure is easy to understand, and one can also use this example to study more complex structures in organic chemistry. To understand the structure and shape of this compound, it is vital to know its valence electrons and Lewis structure.
Contents
CH3OH Valence Electrons
Methanol consists of one carbon atom, three Hydrogen atoms, and one hydroxyl group. To know the total number of valence electrons, we have to know the valence electrons of all the atoms individually:
Carbon has four valence electrons in its outer shell, hence the valence electrons in Carbon= 4.
Hydrogen has only one valence electron, but as there are three Hydrogen atoms in this compound, the total number of valence electrons for Hydrogen = 3*1= 3.
Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule; hence its valency is 6.
Hydrogen attached to the Oxygen in the hydroxyl group has one valence electron; hence its valency is 1.
Total number of valence electrons in CH3OH = 4 + 3+6+1
= 14
Thus the total number of valence electrons in CH3OH ( Methanol) is 14.

Octet Rule
In chemistry, all the atoms tend to become inert by attaining the electronic configuration of the noble gas that has eight electrons in its outer shell. Hence all the atoms tend to form bonds in achieving this configuration and become stable. This rule has some exceptions in chemistry, but majorly, all elements follow this octet rule.
CH3OH Lewis Structure
Lewis dot structure is a pictorial representation of the molecule, it’s bonding with other atoms and the arrangement of atoms in the compound. It helps in knowing the number of bonded electrons, lone pairs, and the compound’s molecular shape. Valence electrons help in drawing this Lewis structure, as all the electrons are shown by using dots, and the straight lines represent the bonds formed between the molecules.
Here in CH3OH,
There are a total of 14 valence electrons in the compound. Carbon has a steric number of 4 as it has four valence electrons in its outer shell. In Methanol, Carbon is the central atom, and all the other atoms are placed around it.
For drawing the structure, you can place four electrons ( as dots ) around the central carbon atom in all four directions. Now all the Hydrogen atoms have one valence electrons, and all these three atoms form a bond with Carbon by sharing one electron of the Carbon atom. To represent these bonds, draw straight lines between three Hydrogen atoms and the central carbon atom.

Hydroxyl group ( OH) shares one valence electron with Carbon, and thus this hydroxyl group forms one bond with the Carbon by sharing its valence electron. There are four valence electrons left in the outer shell of the oxygen atom as it shares one of its six valence electrons with Hydrogen and another one with the Carbon atom. Still, there are four valence electrons on the Oxygen atom that forms two lone pairs of electrons around it. Thus all the valence electrons of the Carbon atoms have now formed bonds, and there are no lone pairs or non-bonded electrons on the central Carbon atom but Oxygen has two lone pairs of electrons.
CH3OH Molecular Geometry
Now that we know the Lewis structure of CH3OH, it is easy to depict the compound’s molecular geometry. While drawing the Lewis structure for CH3OH, you will notice that the Carbon atom will have three bonds with three hydrogen atoms and one bond with the Hydroxyl Group.
As the Carbon has four valence electrons that form the bonds with other atoms, it shows sp3 hybridization.

CH3OH Shape
The hybridization of the central atom ( Carbon ) in CH3OH is sp3, which means that it should form a tetrahedral shape, but it doesn’t form this shape exactly. The shape of Methanol is bent because the hydroxyl group ( OH) contains two lone pairs of electrons, which cause the repulsion between the bonded pair of electrons and the non-bonded pair of electrons in the compound. These repulsion forces lead to information of a bent structure.
According to some theories, it is also believed that CH3OH has two geometric centers, one for the Carbon atom and another for the Oxygen atom in the hydroxyl group. The central carbon atoms form four sigma bonds and have no lone pairs, which results in the formation of a tetrahedron. Simultaneously, the Oxygen atom forms two sigma bonds and two lone pairs of electrons, which causes a bent in the bond angle due to the repulsion forces. Thus Oxygen has a bent tetrahedral shape, resulting in the bent shape of Methanol.
Concluding Remarks
The structure of Methanol or CH3OH is comparatively easy to study as the valency of the central Carbon atom is fully satisfied, and there are no lone pairs on the carbon atom. The atom shares three of its four valence electrons with Hydrogen atoms and rests one electron with the hydroxyl group. Central Carbon atom has sp3 hybridization and a bent molecular shape due to the repulsion between lone pairs on Oxygen and the bonded pairs in the molecule.