 The chemical formula C3H6O represents acetone. This compound is also referred to as propanone or, in some texts as Propan-2-one. Acetone is considered to be the simplest form of Ketone. This compound is colorless, flammable, and is pungent smelling. It boils at temperatures of approximately 56° C.
The chemical formula C3H6O represents acetone. This compound is also referred to as propanone or, in some texts as Propan-2-one. Acetone is considered to be the simplest form of Ketone. This compound is colorless, flammable, and is pungent smelling. It boils at temperatures of approximately 56° C.
Acetone is an important organic solvent, and it is used in the dissolution of fats, resins, and many other organic substances. It is also readily miscible in water.
Acetone is widely used in various applications, so much so that over 6 million tons of the compound are produced annually. Acetone is used as an industrial solvent and is a key component in the production of plexiglass. In the latter, acetone is used as an intermediate. Acetone is converted to acetone cyanohydrin, which is further synthesized to obtain methyl methacrylate (plexiglass).
Acetone is also used medically as a solvent and an anti-convulsing agent. In daily life, we can find this compound in products such as nail polish.
Acetone is produced by the Cumene process, where benzene is alkylated with propylene to produce isopropylbenzene (cumene) which is then oxidized by air to obtain acetone and phenol.

The body is also said to produce small amounts of acetone as it undergoes ketosis. Acetone is extremely flammable and burns yellow. It can cause flash fires and explosions if oxidized.
Some of the properties of C3H6O are shown in the table below:
| Name of the molecule | C3H6O (Acetone) |
| No. of valence electrons | (3 x 4) + (6 x 1) + 6 = 24 valence electrons |
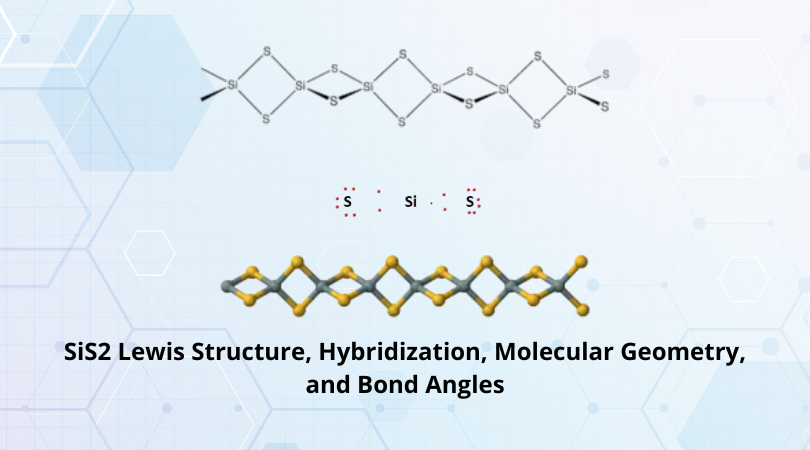
| Hybridization of the central atom | sp2 |
| Bond Angles | 116° |
| Molecular Geometry of C3H6O | Trigonal Planar Molecular Geometry |
Contents
C3H6O Valence Electrons
In simple terms, the valency of an element is defined as its combining capacity, i.e., the number of bonds it can form.
Valence electrons help facilitate this chemical bonding. They are present in the outermost shells of the atom, where the force of attraction from the nucleus is the weakest. This allows them to break away to help form chemical bonds between elements.
Lewis structures make use of valence electrons to help represent a schematic state of a particular molecule.
Let us now calculate the number of valence electrons available to us in C3H6O.
Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, the three Carbon atoms contribute 4 x 3 = 12 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the six Hydrogen atoms contribute 1 x 6 = 6 valence electrons.
Being in group 6 of the periodic table, oxygen has six valence electrons and has a valency of -2. Oxygen’s electronic configuration is 1s22s22p4.
Therefore, the lone Oxygen atom present in the molecule contributes 6 x 1 = 6 valence electrons.
Therefore, the total number of valence electrons in Acetone (C3H6O):
12[C] + 6[H] + 6[O] = 24 Valence Electrons
C3H6O Lewis Structure
The Lewis structure represents a schematic arrangement of the atoms in a molecule. It is a 2-D structure that shows chemical bonds, valence electrons, lone pairs and electronic shapes.
We’ve already completed the first step, i.e., to calculate the number of valence electrons available. As discussed in the previous section, there are 24 valence electrons available to us.
The Acetone molecule comprises three carbon atoms, six hydrogen atoms, and a lone oxygen atom. This compound is a Ketone group.
As such, one of the carbon atoms will be in the center. This carbon atom forms chemical bonds with the surrounding oxygen and carbon atoms. C2 (carbon 2) will act as the central atom of the C3H6O molecule.
Two valence electrons are used to form chemical bonds between atoms. This is represented in the figure below.

The next step is to fulfill the octet requirements for each of the atoms in the acetone molecule. We can observe from the previous figure that C1 and C3 have eight valence electrons. Their octets have been filled. The same is true for each of the hydrogen atoms. Hydrogen requires only two valence electrons to fill its outermost shell.
Now, we can address the octet requirements for the central carbon atom and the oxygen atom. We have six valence electrons remaining. As per the rule, these six valence electrons go to the outer atoms first, meaning the oxygen atom obtains these electrons. This is shown in the figure below.
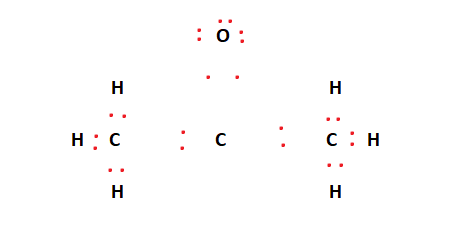
However, C1 – the central atom, has only six valence electrons and needs two more. As such, C1 forms a double bond with the oxygen atom. This is shown in the figure below.
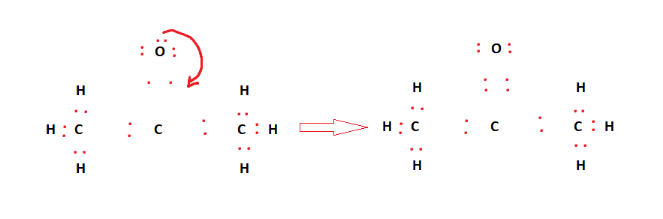
In the above figure, all of the constituent atoms have filled outermost shells. This means that the structure is stable. Therefore, the final Lewis structure for acetone [C3H6O] is given below:

C3H6O Polarity
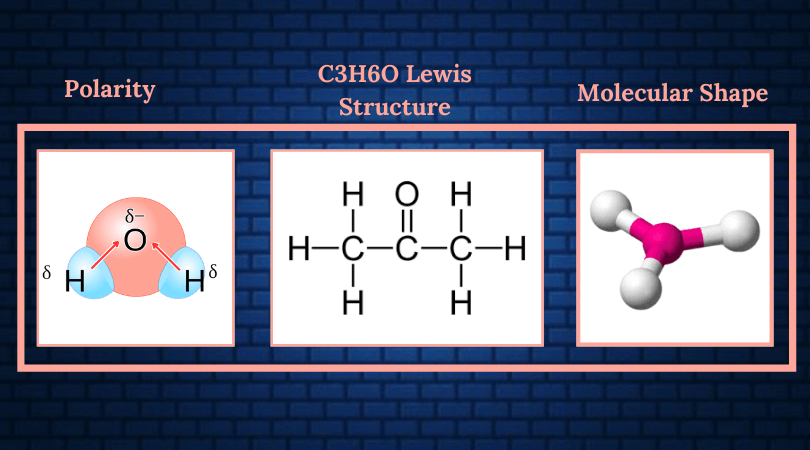
Polarity is the phenomenon where charges are separated to form an electric dipole moment. Unsymmetrical geometry, the nature of the bonds, and the presence of a dipole moment are some of the factors that make a molecule polar.
Acetone is said to be a polar molecule. To understand why this is the case, we must dive deeper into the factors affecting chemical polarity, keeping acetone in mind.
Electronegativity and Bond Nature
The electronegativity of atoms in a molecule helps determine the nature of the chemical bond. The difference in electronegativity between two atomic groups in a compound leads to the formation of polar regions. These polar regions then possess partial electropositive or partial electronegative charges.
According to the Pauling scale, if the difference in electronegativity lies between 0.5 and 2.0, the bonds are polar in nature. There are two different chemical bonds that we pay attention to here- the first of which is the C-O bond and the second, the C-H bond.
The difference in electronegativity between Carbon and Oxygen is 0.89, making this bond slightly polar in nature.
The same cannot be said for the C-H bond since the difference there is only 0.35.
This indicates that the hydrogen ends of acetone are slightly electropositive while the oxygen is electronegative.
Molecular Shape and Symmetry
The molecular shape of the acetone molecule is trigonal planar due to it having an AX3 structure.
Since there is a difference in electronegativity, the charges are distributed unevenly, with the hydrogen regions being more electropositive, as shown in the figure below.
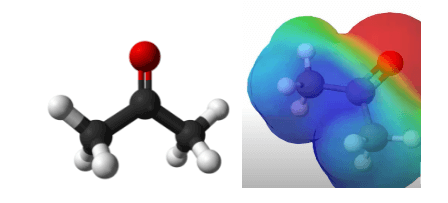
Net Dipole Moment
Due to the significant differences in electronegative charge, there is a net dipole moment going through C2 directed towards the oxygen atom. This confirms that acetone is a polar molecule.

CONCLUDING REMARKS
Let’s quickly summarize the salient features of C3H6O
- C3H6O consists of three carbon atoms, six hydrogen atoms, and a lone oxygen atom. It is the simplest ketone group.
- The central carbon atom forms covalent bonds with its neighbors while also forming a double bond with an oxygen atom. The outer carbon atoms are bonded to the hydrogen atom.
- C3H6O is a polar molecule due to a net dipole moment being present.
- C3H6O has a Trigonal Planar molecular structure with bond angles of 116°.