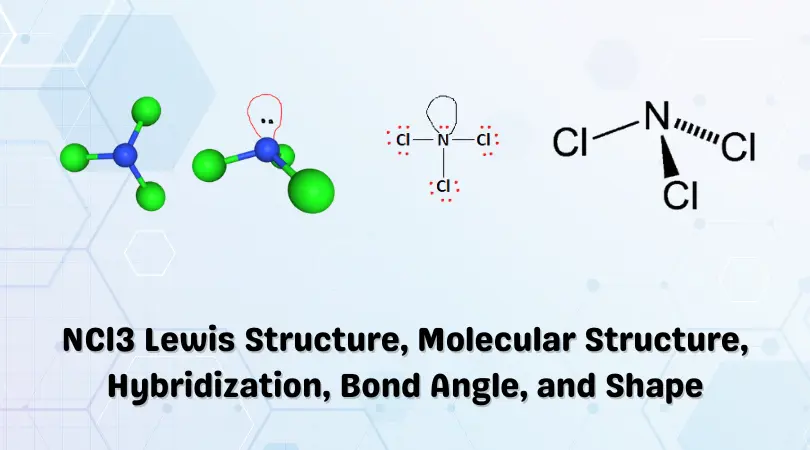
The chemical formula NCl3 represents the inorganic chemical compound Trichloramine. It is also commonly known as Nitrogen Trichloride. It is an oily, yellow liquid with a strong, pungent smell.
Trichloramine is one of many DBPs (Disinfection by-products) present in modern swimming pools. It is formed in small amounts when Chlorine reacts with urea in sweat and urine from swimmers and bathers. Furthermore, it turns into a gas by virtue of its volatile nature.
It is highly explosive and toxic in concentrated amounts [1]. Research into DBPs at swimming pools holds Trichloramine responsible for instances of asthma among swimmers and staff [2]. Trichloramine in the air causes eye irritation, commonly observed after swimming. The distinct smell of Chlorine in pools is attributed to the presence of Trichloramine in the air.
The physical properties of Trichloramine are given below:
| Name of the molecule | Trichloramine (NCl3) |
| No. of valence electrons | (5 x 1) + (7 x 3) = 26 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 107.1° |
| Molecular Geometry of NCl3 | Trigonal Pyramidal |
Contents
NCl3 Lewis Structure
Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. They’re usually the first figures drawn to represent molecules and understand their properties. Dots and lines are used in the Lewis structure to describe electrons and chemical bonds, respectively.
First, the number of valence electrons present in the structure must be determined.
Valence Electrons
Valence electrons are those electrons that lie in the outermost shell of the atom. Here, the force of attraction from the nucleus on these electrons is weak. Thus, valence electrons break free to participate in the chemical bond formation or electron exchange.
Each atom in the molecule contributes a set number of valence electrons depending upon their atomic number and position on the periodic table. These valence electrons are used as building blocks in the Lewis structure.
Trichloramine comprises a single Nitrogen atom and a set of Chlorine atoms. Let us determine the number of valence electrons present in this molecule.
Nitrogen is in group 5 of the periodic table with the electronic configuration 1s22s22p3. Therefore, the lone Nitrogen atom in NCl3 contributes 5 x 1 = 5 valence electrons.
Being in group 7 of the periodic table, chlorine has seven valence electrons. Chlorine’s electronic configuration is given by [Ne]3s23p5. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three Chlorine atoms present contribute 7 x 3 = 21 valence electrons.
Therefore, the total number of valence electrons present in Trichloramine(NCl3) is given by:
5[N] + 21[Cl] = 26 valence electrons
Lewis Structure Assembly
Twenty-six valence electrons are available as building blocks for the Lewis structure. Next, we form a skeletal structure by determining the central atom(s). Nitrogen is the least electronegative atom in the group and, therefore, takes its place as the central atom. The set of Chlorine atoms surrounds the central Nitrogen atom. This is illustrated in the figure below:

The valence electrons slot right in between the atoms to form chemical bonds. This is again shown in the figure below:

The red dots in the structure represent valence electrons. When distributing valence electrons, the aim is to ensure each atom fulfills its outer shell requirements, i.e., adheres to the octet rule. The remaining valence electrons, 20 in this case, are distributed amongst the atoms to fulfill the aforementioned outer shell requirements.
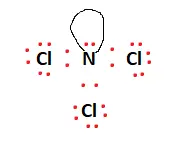
There is a lone pair attached to the Nitrogen atom, and this fulfills the octet requirements of the entire molecule. All valence electrons have been used, and the structural arrangement is now stable. The final Lewis structure is given below:
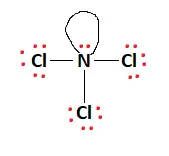
NCl3 Hybridization
Molecular structure and bond formation can be better explained with hybridization in mind. The Hybrid orbitals formed to give a more accurate description of electron regions while also resulting in more stable bonds. An easy way to determine the hybridization of an atom is to calculate the number of electron domains present near it. The bond between atoms (covalent bonds) and Lone pairs count as electron domains.
In this case, the Nitrogen atom in Trichloramine forms three sigma bonds with the surrounding Chlorine atoms. There is also a lone pair attached to the Nitrogen atom. This gives the Nitrogen atom a steric number of 4, i.e., there are four domains attached to it.
Therefore, the Nitrogen atom at the center of Trichloramine has an sp3 hybridization.
NCl3 Angles
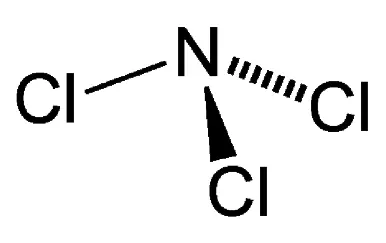
There are three Chlorine atoms surrounding the central Nitrogen atoms. This gives it a Trigonal Planar shape. However, the presence of a lone pair changes the shape into a Trigonal Pyramidal one. The bond angles, in this case, are expected to be 109.5°. According to the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), the lone pair on the Nitrogen atom will repel the atoms around it, pushing it down it further, resulting in bond angles of 107.1°.
NCl3 Molecular Geometry and Shape
The Lewis structure of a compound gives insight into its molecular geometry and shape. From the Lewis structure, it can be observed that Nitrogen is the central atom while the set of Chlorine atoms present in the molecule surround the Nitrogen atom.
According to the VSEPR theory, electron regions on atoms will repel each other as much as possible. This repulsion pushes the atoms apart to give molecular geometry. The presence of three Chlorine atoms presents a Trigonal Planar shape. However, the lone pair attached to the Nitrogen atom repels the Chlorine atoms to give a Trigonal Pyramidal structure.
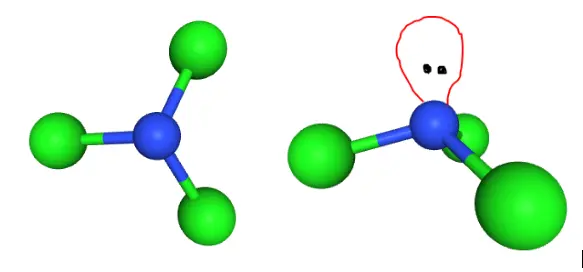
The figures above help visualize the change in molecular geometry due to repulsion from the lone pair on Nitrogen.
We can use the A-X-N method to confirm this.
‘A’ here represents the central Nitrogen atom. Therefore, ‘A’ = 1 in this case.
‘X’ represents the number of atoms bonded to the central atom. In this case, the Nitrogen atom forms three covalent bonds with the set of surrounding Chlorine atoms.
Therefore, X =3 for the Nitrogen atom.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1, and a single lone pair of electrons is attached to the central nitrogen atom.
Therefore, that would give us an A-X-N notation of AX3N for the Nitrogen atom and the molecule as a whole.
From the A-X-N table below, we can determine the molecular geometry for Trichloramine(NCl3).
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
From the above table, it can be observed that an AX3N arrangement corresponds to a Trigonal Pyramidal geometry. Therefore, the Trichloramine molecule possesses a Trigonal Pyramidal molecular geometry.
CONCLUDING REMARKS
Let’s quickly summarize the salient features of Trichloramine
- Oily, Yellow, and pungent-smelling liquid that is extremely toxic and explosive. Found in swimming pools as a DBP (Disinfection by-product) and can cause irritation and respiratory symptoms.
- NCl3 comprises a single, central Nitrogen atom that forms covalent bonds with a set of three surrounding Chlorine atoms. There is also a lone pair attached to the central nitrogen atom.
- The hybridization of the central Nitrogen in Trichloramine is sp3.
- Trichloramine has a trigonal pyramidal molecular geometry with bond angles of 107.1° due to the presence of a lone pair at the central Nitrogen atom.