The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. It is a greenish-yellow crystalline solid with an irritating odor.
It is mainly used as a chlorinating reagent. In a solid state at room temperature, PCl5 exists as an ionic compound with the formula PCl+4PCl–6. It partially dissociates after self-ionization in polar solvents such as Nitrobenzene. Phosphorus Pentachloride is prepared by the dry action of Chlorine on PCl3 in a chlorination reaction.
PCl3 + Cl2 ⇌ PCl5 (ΔH = −124 kJ/mol)
PCl5 undergoes hydrolysis to release hydrogen chloride and oxides of phosphorus. The first oxide released is POCl3. PCl5 is used in both oxidative and substitutive chlorination reactions. Carboxylic acids can be converted to acyl chloride, whereas alcohols can be converted to alkyl chlorides. PCl5 is electrophilic and is also a Lewis acid. PCl5 is a precursor for components used to manufacture Lithium-ion batteries.
The physical properties of Phosphorus Pentachloride (PCl5) are given below:
| Name of the molecule | Phosphorus Pentachloride (PCl5) |
|---|---|
| No. of valence electrons | (5 x 1) + (7 x 5) = 40 valence electrons |
| Hybridization of the central atom | sp3d |
| Bond Angles | 90° & 120° |
| Molecular Geometry of PCl5 | Trigonal Bipyramidal |
Contents
PCl5 Lewis Structure
Chemical bonds and electrons present in a molecule can be accurately represented using schematic diagrams called Lewis structures. The structure uses elementary dots and lines to describe bonds between atoms and valence electrons present. Lewis structures also give insight into the chemical polarity, molecular geometry & bond angles of a molecule.
The first step in obtaining the Lewis structure for a particular molecule is to calculate the number of valence electrons.
Valence Electrons
Each electron in an atom is accounted for and can be found in particular orbitals and subshells depending on their quantum properties. Valence electrons participate in covalent bonding and electron exchange by virtue of their position with respect to the atomic nucleus. They lie in the atom’s outermost shell, where the force of attraction tethering them to the nucleus is at its weakest. This position enables valence electrons to break free to participate in bond formation.
Depending upon the atomic number and position of the element on the periodic table, a set number of valence electrons are made available. The available valence electrons are used to build and define the Lewis structure.
PCl5 comprises a single Phosphorus atom and a set of five Chlorine atoms. Let us now determine the number of valence electrons present in this molecule.
Phosphorus is present in group 5 of the periodic table of elements with the electronic configuration: [Ne] 3s²3p³. Therefore, the single Phosphorus atom in Phosphorus Pentachloride contributes 5 x 1 = 5 valence electrons.
Being in group 7 of the periodic table, Chlorine has seven valence electrons. The electronic configuration of Chlorine is given by [Ne]3s23p5. Therefore, the five Chlorine atoms present contribute 7 x 5 = 35 valence electrons.
Therefore, the total number of valence electrons made available by Phosphorus Pentachloride (PCl5) is given by:
5[P] + 35[Cl] = 40 valence electrons
Lewis Structure Assembly
Phosphorus Pentachloride possesses forty valence electrons, as calculated in the previous section. Next, a skeletal structure is formed by determining the central atom(s). Phosphorus is the least electronegative atom in the molecule and, therefore, facilitates bond formation as the central atom. The set of five Chlorine atoms surrounds the central Phosphorus atom. This is illustrated in the figure below:
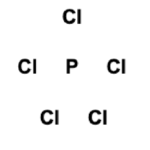
Electrons are placed in between atoms to represent covalent bonds. Ten electrons have been used, as shown in the figure.
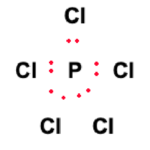
One of the objectives of the Lewis structure assembly is to ensure that atoms in PCl5 fulfill their outer shell requirement while adhering to the octet rule. Therefore, the remaining 30 valence electrons will now be distributed amongst the atoms to meet outer shell requirements.
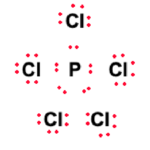
Now that all the valence electrons have been used up, we verify the stability of the structure. Each of the Chlorine atoms now has filled outer shells and is in adherence with the octet rule. However, the central Phosphorus atom has ten valence electrons.
This is unusual but completely stable as an exception to the octet rule. Phosphorus is in period 3 of the periodic table, and the d subshell becomes available, leading to the element having an expanded octet. Therefore, it can be said the Lewis structure is stable. The final Lewis structure is given below:
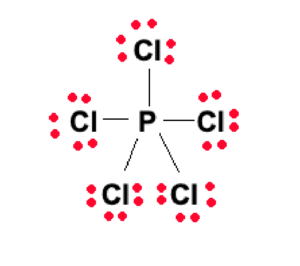
PCl5 Hybridization
We can understand molecular structure and bond formation intuitively through hybridization. The formation of hybrid orbitals gives insight into the probability of electron regions and bond formation. A quick method to determine the hybridization of a molecule is to calculate the steric number or the number of electron domains present near the central atom. The bond between the atoms (covalent bonds) and Lone pairs count as electron domains.
In this case, the central Phosphorus atom in PCl5 forms five sigma bonds with the surrounding Chlorine atoms. This gives the Phosphorus atom a steric number of 5, i.e., five domains are attached. There are no lone pairs.
Therefore, the Phosphorus atom at the center of Phosphorus Pentachloride has an sp3d hybridization.
PCl5 Angles
There are five Chlorine atoms surrounding the central Phosphorus atom. As we continue to add Chlorine atoms, the shape of the molecule changes in accordance with the VSPER theory. At three Chlorine atoms, we get Trigonal Planar molecular geometry. Upon adding the remaining two Chlorine atoms, we end up with a Trigonal Bipyramidal geometry with bond angles of 90° & 120°.
PCl5 Molecular Geometry and Shape
The Lewis structure of a compound gives specific insight into its molecular geometry and shape. In PCl5, Phosphorus is the central atom, and five Chlorine atoms surround it.
According to the VSEPR theory, electron regions in atoms will repel each other as much as possible. This repulsion pushes the atoms apart to give a semblance of molecular geometry. Adding Chlorine atoms to the central Phosphorus atom leads to electronic repulsion, which determines molecular geometry. Three Chlorine atoms give a Trigonal Planar shape. The addition of two more Chlorine atoms results in Trigonal Bipyramidal molecular geometry.
The A-X-N method, based on steric numbers, can be used to confirm this.
‘A’ here represents the central Phosphorus atom. Therefore, ‘A’ = 1.
The number of atoms bonded to the central atom is represented by ‘X.’ In this case, the Phosphorus atom forms five covalent bonds with the surrounding Chlorine atoms.
Therefore, X =5 for Phosphorus Pentachloride.
The number of lone pairs associated with the central atom is given by ‘N.’ Here, N = 0, as no lone pairs are attached to the central Phosphorus atom.
Thus, the A-X-N method leaves us with a notation of AX5 for Phosphorus Pentachloride.
From the A-X-N table below, we can ascertain the molecular geometry for PCl5.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyramidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
We can observe from the above table that an AX5 notation corresponds to a Trigonal Bipyramidal geometry. Therefore, the Phosphorus Pentachloride molecule possesses a Trigonal Pyramidal molecular geometry.
Concluding Remarks
Let’s quickly summarize the salient features of PCl5
- Greenish-yellow crystalline solid with an irritating odor. Mainly used as a chlorinating reagent.
- PCl5 comprises a single, central Phosphorus atom that forms covalent bonds with five surrounding Chlorine atoms.
- The hybridization of the central Phosphorus atom in PCl5 is sp3d.
PCl5 has a trigonal bipyramidal molecular geometry with bond angles of 90° & 120°.