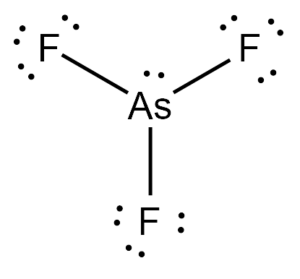
The chemical formula AsF3 represents the compound Arsenic Trifluoride. It exists as a colorless liquid at room temperature and decomposes in water. It is also soluble in alcohols, ethers, and ammonia solutions.
Arsenic Trifluoride is formed when Hydrogen Fluoride reacts with Arsenic Trioxide. This reaction is shown below:
6HF + As2O3 → 2AsF3 + 3H2O
Arsenic Trioxide also reacts with Sulfuric acid and Calcium Fluoride to give AsF3.
Arsenic Trifluoride continues to be used as a fluorinating agent to convert non-metallic chlorides to fluoride-based compounds. It is also used as a catalyst, an ion implantation source, and a dopant.
Arsenic Trifluoride is said to be polar. Let us understand why this is the case. To determine the polarity of the NH2- ion, we must first account for its properties. These include its electronegativity, its molecular geometry, and its resulting dipole moment if any.
AsF3 Electronegativity and Bond Nature
Electronegativity is a significant factor affecting chemical polarity. The difference in electronegativity between two atomic groups in a compound leads to the formation of polar regions. These polar regions then possess partial electropositive or partial electronegative charges.
We look to the periodic table to determine the difference in electronegativity between Arsenic and Fluorine. In this case the difference is 3.98 [F] – 2.18 [As] = 1.80.
The figure obtained above represents the disparity in electronegativity between Fluorine and Arsenic. This is indicative of polar regions being created on account of this.
The Pauling scale helps simplify things in terms of determining the nature of the bonds in a molecule. According to the Pauling scale, if the difference in electronegativity lies between 0.5 and 2.0, the bonds are said to be polar in nature.
The difference in electronegativity of the Fluorine and Arsenic atoms is 1.80. This figure falls in the range specified above, and hence, the As-F bond can be classified as polar.
This polar nature is indicative of chemical polarity being present in AsF3.
AsF3 Molecular Shape and Symmetry
Arsenic Trifluoride comprises three Fluorine atoms bonded to a central Arsenic atom. There is also a lone pair attached to the Arsenic atom.
According to the VSEPR theory, the Fluorine atoms repel each other at angles of 120°. However, the presence of a lone pair on the Arsenic atom pushes the Fluorine atoms downward, giving the molecule a Trigonal Pyramidal geometry with bond angles of approximately 96°.
This makes the general structure of the molecule asymmetric, and the negative charges are not distributed equally, leading to a net dipole moment.
Net Dipole Moment and 1- Charge in AsF3
As mentioned above, there is a significant disparity in electronegativity between Fluorine and Arsenic. This creates a dipole moment that is directed outwards towards the Fluorine atoms since Fluorine is more electronegative.
Furthermore, the molecular geometry of AsF3 is asymmetric, which indicates a net dipole moment.
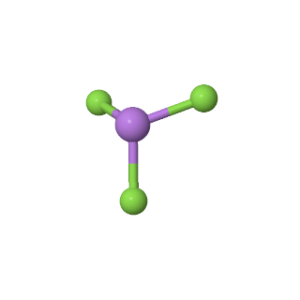
Therefore, the dipole moment vectors direct themselves towards Fluorine and do not cancel each other out due to the presence of a lone pair and the corresponding Trigonal Pyramidal geometry.
Concluding Remarks
Chemical Polarity depends on a few key factors such as Electronegativity, Molecular geometry, and the presence of a dipole moment.
The difference in electronegativity leads to the creation of polar regions. The asymmetrical geometry reinforces the unequal sharing of electrons as Fluorine atoms pull harder due to its higher electronegative charge.
Thus, a net dipole moment is observed whose vectors are directed towards the Fluorine atoms.
All of the above indicates polar nature and proves that Arsenic Trifluoride (AsF3) is polar.