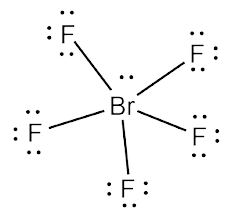
The chemical formula BrF5 represents Bromine Pentafluoride. It is an interhalogen compound and a strong fluorination agent. Interhalogen compounds comprise two or more different halogen atoms and no atoms of elements from other groups.
BrF5 is prepared by treating Bromine with large amounts of Fluorine at temperatures of over 150°C. It reacts violently with water but produces Bromic Acid and Hydrofluoric Acid upon dilution.
It finds use in analytical studies and practical applications. Being a strong fluorinating agent, it reacts with Uranium to form Uranium Hexafluoride. This is then used to enrich Uranium.
We say that Bromine Pentafluoride is polar. Let’s see why that is the case.
To determine the polarity of BrF5, we must first account for its Lewis Structure and properties. These properties include its electronegativity, its molecular geometry, and its resulting dipole moment if any.
Electronegativity and Bond Nature
To determine if the bonds present in BrF5 are polar or non-polar, we look to the periodic table. The difference in charges between the Bromine and Fluorine atoms is 1.02
Now, in accordance with the Pauling scale, this tells us that the Bromine- Fluorine bond in the BrF5 ion is polar.
Molecular Shape and Symmetry
BrF5 comprises Bromine as the central atom with 5 Fluorine atoms surrounding it. It also has two unpaired electrons which att
ach themselves to the central atom, Bromine, as a lone pair.

Bromine Pentafluoride has a square pyramidal molecular geometry. The presence of a lone pair creates a region of negative charge that remains unbalanced. Therefore, it is structurally unsymmetrical making it a polar molecule.
Net Dipole Moment in BrF5
There is a net dipole moment as there is asymmetric charge
distribution due to the presence of the lone pair.

It’s asymmetrical square pyramidal structure also contributes to a net dipole moment.
Conclusion
As shown above, due to its asymmetrical square pyramidal structure and the presence of a lone pair, BrF5 is considered a polar molecule.
The difference in electronegativity between the Bromine and Fluorine atoms also contributes to unequal charge distribution on the central Bromine atom.
Therefore, this proves that BrF5 is a polar molecule.