Chemical bonding is responsible for how substances come to be in the world around us. Unfamiliar atomic species interact with one another to form new substances built on chemical bonds. Here, the energy arrangement of the new substance is at its lowest state. The discovery of the electron and the use of quantum mechanics to describe electron behavior helped scientists understand bond formation. A simple way to understand chemical bonding is to divide types of chemical bonds into three types: ionic, covalent, and metallic bonds.
The position of an element on the periodic table dictates how it participates in chemical bonding. Periodic trends such as atomic size, ionization energy, electron affinity, and electronegativity are key to forming chemical bonds. In addition to these properties, electrons in the valence shell play a significant role in bond formation. Due to various reasons, valence electrons are the ones that take part in the fundamental process of electron sharing and transfer.
The quantum descriptions of chemical bonds are out of the scope of this article. We shall lay special focus on the covalent bond and provide a brief explanation of its definition, types, and characteristics. Examples will also be included for better understanding. We will also discuss the correlation between covalent bonding and molecular geometry.
Contents
Covalent Bonds
What is a covalent bond?
A covalent bond is a chemical bond that results from sharing electrons between two atoms. Atoms share their outermost valence electrons to attain a full octet (complete outer shell). This sharing of electrons between two atoms depends on the physical and chemical properties of the atoms involved in covalent bonding. The electrons being shared between the atoms are called bond pairs. Electrons not involved in bonding are called lone pairs.
Which elements form covalent bonds?
Atoms with low ionization energy and a high affinity for electrons tend to form metallic and ionic bonds to attain a completely filled octet and, thus, chemical stability. When ionization energy is high among the atoms in a compound, sharing electrons forms a covalent bond.
Elements lying towards the right of the periodic table (high ionization energies, small size & high electron affinity) tend to form covalent bonds. Covalent bonds can also be formed when electrons are shared between atoms of the same kind. Molecules such as H2 and C60 are prominent examples.
How are covalent bonds formed?
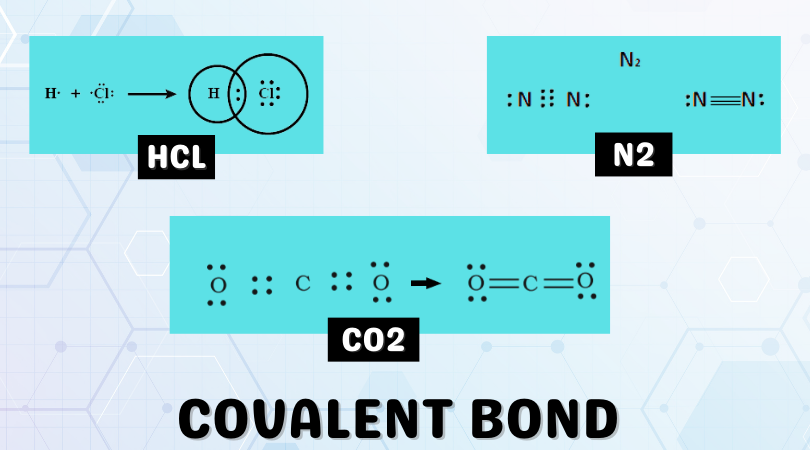
We can use Lewis’ set of conventions and Lewis structures to describe how covalent bonds are formed. Atoms in a molecule lean towards attaining noble gas configuration, i.e., a completely filled s-orbital, and p-orbital. This is known as the octet rule, as eight atoms are required to fill the s and p orbitals. Let us use Hydrogen Chloride (HCl) to illustrate covalent bonding.

The above image represents the Lewis structure of Hydrogen Chloride (HCl). The two atoms, Hydrogen and Chlorine, are denoted by their chemical symbols. Red and black dots represent the valence electrons. We can observe the shared pair of electrons as the red dots in between the atoms- this is the bonding pair. The dots(electrons) around the Chlorine atom are lone pairs and play no part in covalent bonding.
Both Hydrogen and Chlorine atoms attain noble gas configuration and fulfill the octet rule as a result of covalent bonding. The bond pair of electrons allows each HCl molecule to attain stability (lowest energy state). Lewis structures can also describe more complex covalent bonds involving resonance, hypervalence, and double/triple bonding. They also give insight into chemical polarity and electric dipoles formed in some molecules. We will look at the types of covalent bonds in the next section.
Types of Covalent Bonds
Covalent bonds can be divided into the following based on the number of bonding pairs:
- Single Bond
- Double Bond
- Triple Bond
Single Bond
A single covalent bond is formed when only one pair of electrons participates in the covalent bond between two atoms. It is represented by a single line, as the above figure shows Hydrogen Chloride. The single bond is weaker and has less density than a double or triple bond, but it is more stable owing to its low reactivity.
Example: A prime example of a single bond at play is the Hydrogen Chloride compounds mentioned earlier. Hydrogen and Chlorine each attain their octets through a single covalent bond. Each atom contributes a single electron to the bonding pair, thus forming a single bond.

Double Bond
A double covalent bond is formed when two pairs of electrons (two pairs = four electrons) participate in the covalent bond between two atoms. It is represented by two horizontal lines between the atoms in a Lewis structure. A double bond between atoms is much stronger than a single bond but is less stable due to relatively higher reactivity than a single bond.
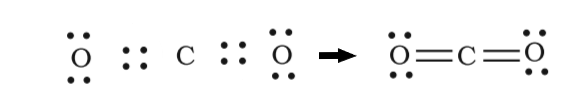
Example: The Carbon Dioxide molecule possesses two double bonds. The Carbon atom has four valence electrons, while the two oxygen atoms have six valence electrons. Carbon requires four electrons to complete its octet and thus forms a double bond with each Oxygen in the molecule. Oxygen contributes two electrons to the molecule and also attains its octet. This is illustrated in the figure below. A double bond can also be observed in the Oxygen (O2) molecule.
Triple Bond
A triple covalent bond is formed when three pairs of electrons (three pairs = six electrons) participate in the covalent bond formed between two atoms. It is depicted by three horizontal lines between the atoms and is the least stable of all covalent bonds, making it vulnerable to species with high electron affinity.
Example: The Nitrogen atom, having five valence electrons, contributes three valence electrons to form three electron pairs for sharing during the formation of the nitrogen molecule. The Nitrogen atoms in the molecule share a triple bond.
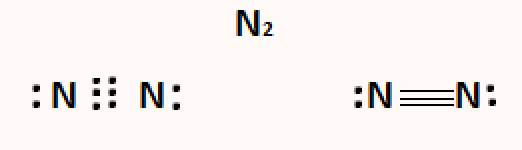
Not all bonds are made equal. A difference in electronegativity leads to unequal sharing of electrons pulling the pair closer to the more electronegative atom in the bonding pair. This results in polar covalent bonds and nonpolar covalent bonds.
Polar Covalent Bonds
A polar covalent bond is created when there is a significant difference in electronegativity between the atoms participating in covalent bonding. The atom with higher electronegativity has a stronger pull on the shared pair of electrons resulting in the shared pair being closer to this atom. A net electrostatic potential is generated, making the molecule polar.
Example: Water (H2O)
Non-polar Covalent Bonds
This type of bond is created when there is zero difference in electronegativity between the bonding atoms. Nonpolar covalent bonds occur in gas compounds such as H2 and N2.
Characteristics of Covalent Bonds:
- Covalent bonds are stable and do not break spontaneously.
- Covalent bonds have relatively lower melting and boiling points than ionic bonds.
- Covalent bonds do not dissolve well in polar solvents such as water.
- Covalent bonds help give insight into the molecular shape and polarity of the molecule.
- Compounds with covalent bonds usually have lower enthalpies of vaporization and fusion.
- Compounds with covalent bonds do not conduct electricity, unlike those with metallic bonds.
- Most compounds with covalent bonds can be described using Lewis structures and follow the octet rule.
- A covalent bond normally contains an energy of about ~80 kilocalories per mole (kcal/mol).
- There are three types of covalent bonds: single, double, and triple. Covalent bonds can also be classified as polar and nonpolar.