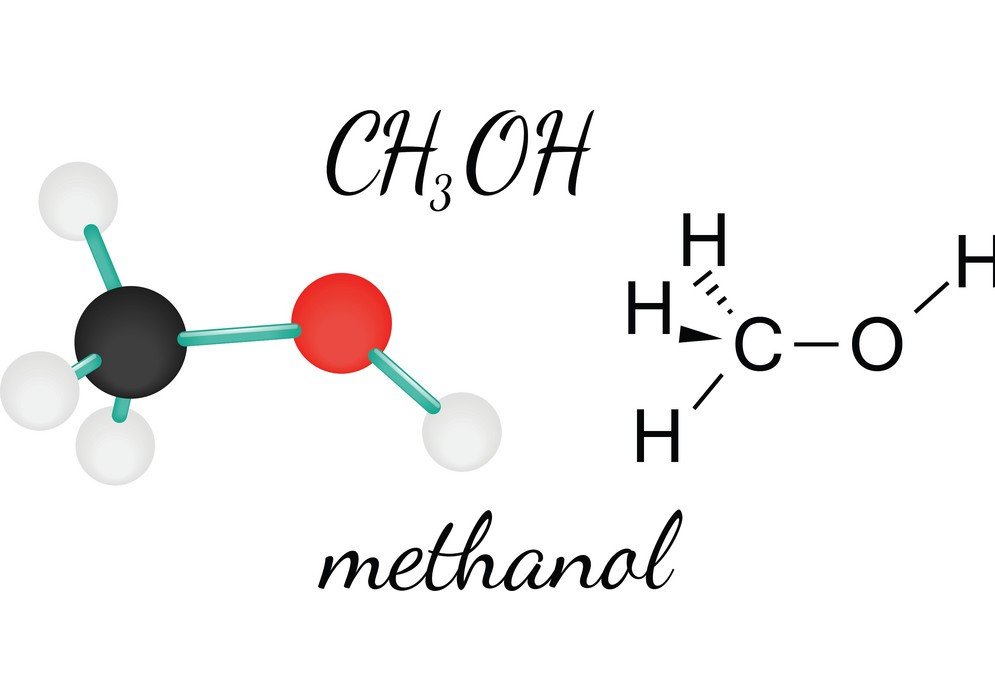
CH3OH or Methanol is a flammable, colorless, and volatile liquid that has a distinctive alcoholic odor. By studying its Methanol, one can get to know the molecular shape, bond angle, and polarity of the molecule. The polarity of CH3OH is one of the vital characteristics as it helps in knowing the other properties of the compound, such as its solubility, electric charges, and much more. To understand Methanol’s polarity, let us first look at the bond angles and the arrangement of the atoms in the CH3OH molecule.
CH3OH Bond angles
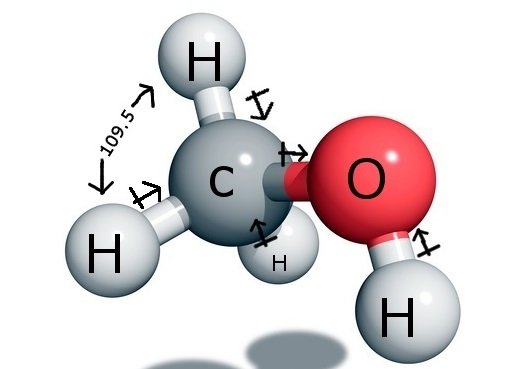
The central Carbon atom forms four bonds in the compound, three with the Hydrogen atom and one with the hydroxyl ( OH) group. And as this carbon atom has an sp3 hybridization and forms a tetrahedron shape, it has the bond angles of 109.5 degrees with its bonding atoms.
In contrast, Oxygen forms one sigma bond but has two lone pairs, so there is a bent in its bond angle due to bonded pair-lone pair repulsion forces. This decreases the bond angle to 104.5 degrees.
Thus Carbon has a bond angle of 109.5 degrees with all the three Hydrogen atoms and a bond angle of 104.5 degrees with the hydroxyl group.
Is CH3OH Polar?
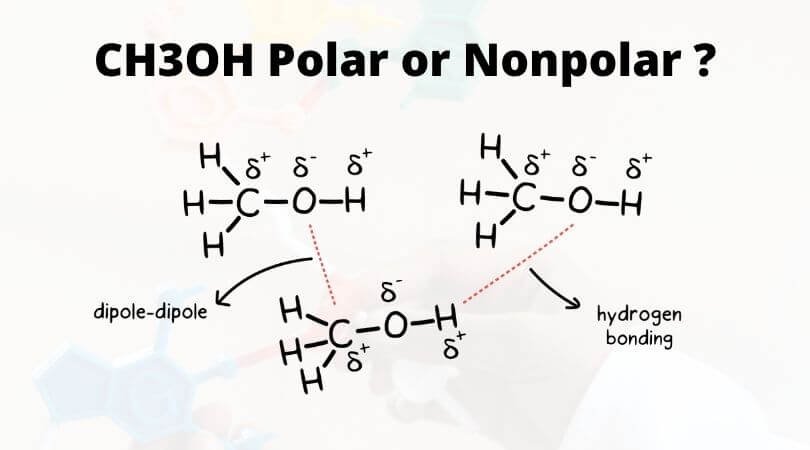
The polarity of any given compound depends on the net dipole moment on the compound. This net dipole moment can be known by noticing the electric charges on the atoms in the molecule. Here both Carbon and Oxygen atoms ( which are considered as geometric centers for this compound) in Methanol are electronegative atoms. But Oxygen is more electronegative than Carbon or hydrogen in the compound.
CH3OH cannot be non-polar because there is a difference in electric charges among the atoms in the methanol molecule. Oxygen has more electron density due to its two lone pairs of electrons. This causes a net dipole pointing towards the Oxygen atom, making CH3OH polar.
Apart from the electric charges, the molecule of CH3OH is asymmetrical, which cancels out the possibility of non-polarity. A non-polar molecule has a symmetrical structure, as the dipole-dipole moment is canceled out. But as there is a bent in the shape of Methanol, it leads to the formation of an asymmetric structure resulting in the net electric dipole moment’s negative end towards the Oxygen atom. Thus CH3OH is a polar molecule.
Now that we know the polarity of the CH3OH molecule, let us go through some of its physical properties:
- The boiling point of Methanol ( CH3OH) is 64.7 °C.
- The melting point of Methanol is -97.6 °C.
- The molecular weight of Methanol is 32.04 g/mol.
- It is a polar solvent and is also known as wood alcohol because it was once produced by the distillation of wood.
- The smell of this compound is on the sweeter side as compared to ethanol.
Uses of CH3OH
- Methanol is also used for producing hydrocarbons and for the synthesis of other chemicals such as formaldehyde.
- It is used as an anti-freeze in pipes.
- CH3OH is also used as a gasoline additive in several countries due to its low melting point.
- The compound is also used as a fuel in internal engine combustion.
- Many pharmaceutical companies use pure Methanol for the synthesis of other compounds.
I hope this article helps you understand the polarity of the molecule and its physical properties and uses. CH3OH is a polar molecule as the dipole-dipole moment is not canceled due to its asymmetric shape.



Hii, i m abhishek tiwari, really it’s very helpful article for students….. thenk for this.
Your Welcome