Chloromethane or Methyl chloride having a molecular formula of CH3Cl is an organic compound. It is an odorless and transparent gas that was initially used as a refrigerant. Later it was found that this gas is toxic and can harm the central nervous system of humans. Although it is no longer used as a refrigerant, Chloromethane has many uses and applications in several chemical and pharmaceutical industries.
To understand its chemical properties and physical properties, one needs first to know the Lewis structure and molecular geometry of CH3Cl. And to help you with understanding its structure in-depth, I will help you to make its Lewis structure step-by-step in this blog post.
But before looking at that, let us first discuss the valence electrons present in this compound as these electrons are the ones that form bonds.
Contents
CH3Cl Valence electrons
If you have been studying chemistry for some time, then you might be aware of the “octet rule.” This rule states that atoms bond with each other atoms such that they have eight electrons in the last valence shell. This happens because it tries to achieve the same valence electron configuration as inert gases. The atoms that have complete octets or rather suffice the octet rule become inert and non-reactive. There are some exceptions to this octet rule, e.g., Hydrogen.
These valence electrons are the ones that participate in the bond formation. So let us have a look at the total number of valence electrons for CH3Cl.
Total no of valence electrons of CH3Cl = Valence electrons of Carbon + Valence electrons of Hydrogen + Valence electrons of Chlorine
Valence electrons of Carbon: 4 electrons
Valence electrons of Hydrogen: 1 * 3 = 3 electrons ( as there are three hydrogen atoms, we will consider valence electrons of all the Hydrogen atoms )
Valence electrons of Chlorine: 7 valence electrons
Total no of valence electrons of CH3Cl= 4 + 3 +7
=14
Chloromethane ( CH3Cl) has 14 valence electrons.
CH3Cl Lewis structure
Now that we know the total number of valence electrons in CH3Cl, we can now draw a Lewis structure for the same. This structure helps understand the arrangement of valence electrons around the individual atoms along with the bonds they form. In Lewis structure, we use dots to represent electrons and lines to show bonds formed between two atoms.
Here we have three types of atoms in CH3Cl: Carbon, Hydrogen, and Chlorine. Out of all these atoms, Carbon is the least electronegative one, and hence we will place it in the central position. Chlorine is the most electronegative atom.
So place the Carbon atom in the center and draw four dots around it like this:
Now that we have placed the Carbon atom, let’s put other atoms. As there are three hydrogen atoms, we will first put these atoms around the Carbon atom. Place three H atoms with one dot around the central atom.
As we have already place carbon and Hydrogen atoms, we just have to place a Chlorine atom in this structure. Place it on the forth side of the Carbon atom like this
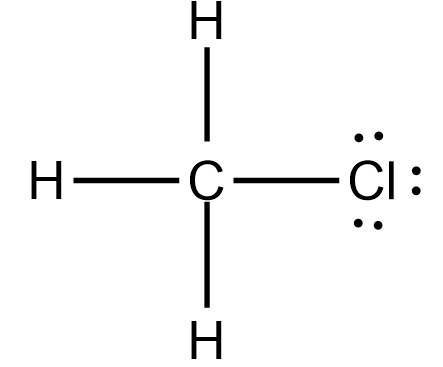
Now, if you see closely, the Carbon atom is sharing four electrons with three hydrogen atoms and a Chlorine atom. As the Carbon atom needs 4 electrons to complete its octet, all the valency is satisfied, and it now has eight electrons in its valence shell.
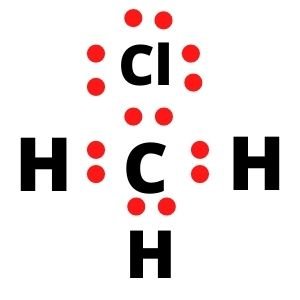
Similarly, each Hydrogen atom needs one electron, which they share with the central Carbon atom, and hence their outer shell is also completed. Due to their sharing of electrons, there is a single bond between C and H atoms.
In contrast, the Chlorine atom also completes its octet as it shares an electron with the Carbon atom. Carbon and Chlorine form a single bond as they share one electron to complete each other’s octet. Hence all the valence electrons are used up, and there are four single bonds in the Lewis structure of CH3Cl.
CH3Cl Hybridization
One can find the hybridization of any given molecule by using this simple formula:
Hybridization = No. of bonds + lone pairs at the central atom
= 4 + 0 ( there are no lone pairs in CH3Cl as there is symmetric distribution of electrons)
=4
The carbon atom has an electronic configuration of 1s22s22p2 in its ground state and has when it is in an excited state; the configuration is 1s22s12p3. Here the electrons shared by the Carbon lead to the formation of four hybridized orbitals, which include one s-orbital and three p-orbitals. All the four electrons are arranged in these hybridized orbitals, making the hybridization of this molecule sp3.
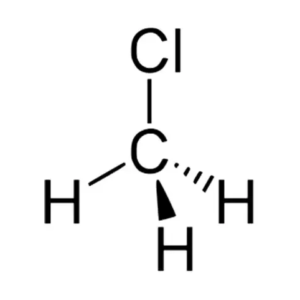
CH3Cl Molecular Geometry
The molecular geometry of any given molecule is based on the number of atoms involved and the bonds formed in the structure. VSEPR theory or Valence electron shall pair repulsion theory is the concept we use to determine the molecule’s shape. This theory states that a molecule takes a shape in which two negatively charged centers are as far from each other as possible ( both bonded and non-bonded pairs of electrons). The theory states that this happens to avoid repulsive forces. According to the VSEPR theory, molecules having a structure similar to AX4, where a molecule has four negatively charged centers, will take a tetrahedral shape.
Also, it has bond angles of 109.5, which corresponds to its molecular geometry. Hence, Chloromethane has a tetrahedral molecular geometry to avoid the repulsive forces and separating the bonded electrons.
Concluding Remarks
To summarize, we can say the following for a single molecule of Chloromethane.
- It has 14 valence electrons, and all of them participate in forming bonds.
- In the Lewis structure of CH3Cl, Carbon is at the central position and all the other atoms around it.
- The bond angles of Carbon with Hydrogen and Chlorine atoms are 109.5 degrees.
- This molecule has a tetrahedral shape, and the central carbon atom has sp3 hybridization.