Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl. Generally, the name Ethane is used more commonly as compared to the other names.
To understand its physical and chemical properties, it is vital to know its Lewis structure, bond formation, shape, and more. Keep reading this blog post to find all the details for Ethane’s Lewis dot structure, its molecular geometry, and hybridization.
| Name of molecule | Ethane ( C2H6) |
| No of Valence Electrons in the molecule | 14 |
| Hybridization of C2H6 | sp3 hybridization |
| Bond Angles | 109.5 degrees |
| Molecular Geometry of C2H6 | Trigonal Pyramidal |
Contents
C2H6 Valence Electrons
Ethane has two atoms of Carbon and six atoms of Hydrogen. To know the molecule’s Lewis Structure, it is vital to know the total number of valence electrons of Ethane.
Carbon has four valence electrons, and each Hydrogen atom has one valence electron.
Total number of valence electrons in Ethane – Valence electrons of Carbon + Valence electrons of Hydrogen
= 2* 4 + 6*1 ( as there are two carbon atoms and six hydrogen atoms we will consider all of them to get the total number of valence electrons)
= 14
Hence there are 14 valence electrons in Ethane.
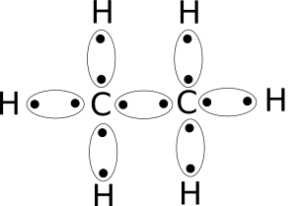
C2H6 Lewis Structure
Lewis structure helps with understanding the placement of atoms in the structure along with its valence electrons. The individual atoms with all their valence electrons are shown in this structure to know the bond formation, molecular geometry, and shape of the molecule.
And as we have the total number of valence electrons now, we can start drawing the Lewis dot structure of Ethane.
Both the Carbon atoms will be placed in the centre as Hydrogen atoms can never be in the central position. So put both the Carbon atoms along with their four valence electrons each like this.
Now place all the Hydrogen atoms around the Carbon atoms along with their valence electrons.
After placing all the atoms, you might notice that each Hydrogen atom needs one electron to attain a stable structure. Similarly, Carbon needs four more electrons to complete its octet.
Each Carbon atom forms bonds with three Hydrogen atoms and shares electrons. Due to this each Hydrogen atom now has two electrons in its outer shell which makes it stable.
Both carbon atoms also form single bonds with each other and share their electrons to complete their octet. Here in Ethane, Carbon forms bonds with Hydrogen atoms which helps with these atoms to attain a stable structure and at the same time form bond with other Cabron atom to complete their octet.
All the valence electrons are used up to make the molecule’s stable structure, hence it doesn’t have any lone pairs or nonbonding pairs of electrons. The arrangement of the electrons and atoms is symmetric in this molecule.
This is the Lewis Dot structure of C2H6 using up all the fourteen valence electrons.
C2H6 Hybridization
During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. As a result, four orbitals that is 1s, px, py and pz orbitals are hybridized in each Carbon atom. Amongst these hybrid orbitals, one hybrid orbital will overlap with the 1s-orbital of the Hydrogen atom that produces the sigma bond between a Hydrogen and Carbon atom. The formation of such hybridized orbitals results in sp3 hybridization.
Thus, C2H6 is sp3 hybridized.
C2H6 Molecular Geometry
If you look at the arrangement of atoms in the molecule, you will notice that all the Hydrogen atoms are arranged around Carbon atoms in the tetrahedral geometry. Hence C2h6 has a tetrahedral molecular geometry.
C2H6 Bond Angles
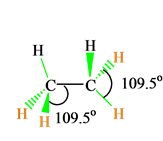
The molecules with a tetrahedral molecular geometry have bond angles of 109.5 degrees, which are typically affected by lone pairs of electrons. Lone pair of electrons can change the bond angles due to their repulsive forces, but here in C2H6, as there are no lone pairs in the molecule, the bond angles in C2H6 is 109.5 degrees.
C2H6 Shape
As mentioned above, the molecule has a tetrahedral geometry without any lone pairs. However, the electron clouds that are on both the Carbon atoms will repel each other. These forces lead to the formation of a trigonal pyramidal shape for this molecule.
Thus C2H6 has a trigonal pyramidal shape.
Concluding Remarks
Ethane is one of the simplest hydrocarbons due to its structure. It only has two carbon atoms and symmetric distribution of molecules with lone pairs. One can easily understand the Lewis dot structure of this molecule. Ethane is widely used in the petrochemical industry and for the production of ethylene. It is also used in the preparation of other chemicals such as adhesives, paints, etc.
I hope this article helps you understand the Lewis structure of the C2H6 with ease.
Let us know which other molecule’s Lewis structure you would like to know in the comments below.



THANK YOU SO MUCH THIS WAY VERY HELPFUL