Oxygen Difluoride was first reported in 1929 while carrying out electrolysis of potassium chloride with hydrofluoric acid in the presence of water in little quantities. This molecule’s chemical formula is OF2 as it contains one atom of Oxygen and two atoms of Fluorine. It’s easy to understand the Lewis structure of this molecule as it has only two types of atoms in the molecule.
| Name of molecule | Oxygen Difluoride ( OF2) |
| No of Valence Electrons in the molecule | 20 |
| Hybridization of OF2 | sp3 hybridization |
| Bond Angles | 109° 27′ |
| Molecular Geometry of OF2 | Bent |
For finding out the molecular geometry and hybridization of the molecule, it is vital first to know the Lewis Structure of OF2. The Lewis structure of any molecule is a pictorial representation of the arrangement of valence electrons around individual atoms along with the bonds they form. This structure helps in determining other properties of the molecule. So here we will first look at the Lewis Structure of Oxygen Difluoride and then check out other properties of this molecule.
Contents
OF2 Valence Electrons
To find out the Lewis dot structure of any molecule, one should know its total number of electrons. Here we will find out the total number of valence electrons for OF2 by considering the valence electrons of all the atoms.
Total valence electrons in OF2 – Valence electrons of Oxygen + Valence electrons of Fluorine
Oxygen has six valence electrons in its outer shell.
Each fluorine atom has seven valence electrons, but as there are two Fluorine atoms, we will multiply the number by 2. So we have 14 valence electrons from Fluorine atoms.
= 6 + 7*2
= 20 valence electrons
Thus, there are 20 valence electrons available for OF2 that help the atoms to form bonds.
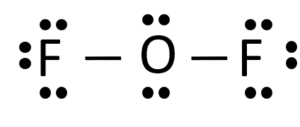
OF2 Lewis Structure
Now that we know the total number of valence electrons for OF2, we can make the Lewis dot structure of OF2. Remember that all atoms follow the octet rule, which mentions that an atom that wants to attain a stable structure like inert gases must have eight valence electrons in its outer shell. However, there are many exceptions to this rule, but we always keep this rule in mind while drawing the Lewis structure for any molecule.
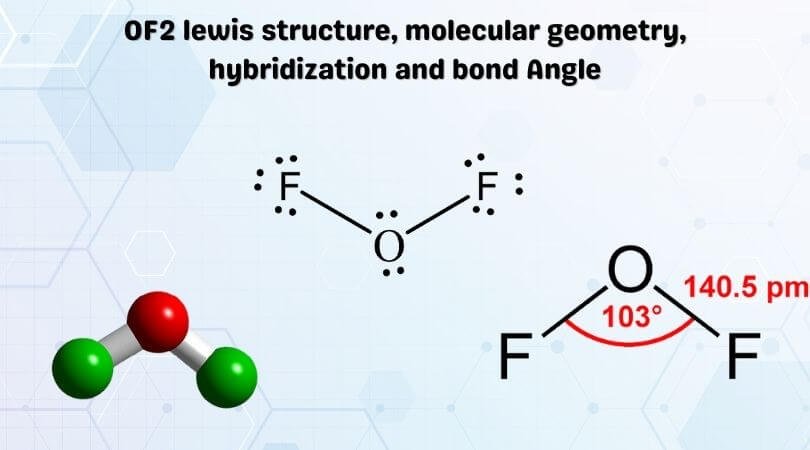
In OF2, the Oxygen atom will acquire the central position, and both the Fluorine atoms will go on the sides like this. As Oxygen is less electronegative than the fluorine atom, it will be placed in the centre.
Once you have placed all the atoms in this manner, start putting the valence electrons of the individual atom around them. For example, Oxygen has six valence electrons, so draw six dots around the atom.
Now each Fluorine atom forms a single bond with an Oxygen atom by sharing electrons. Every single bond will use up to 2 electrons.
Fluorine atoms will complete their octet by sharing electrons as now they will have eight electrons in their outer shell. However, Oxygen atom is left with two pairs of nonbonding pairs of electrons.
The Lewis Structure of OF2 will have single bonds between O-F, with Oxygen atom in the centre. There are two lone pairs of electrons on the central atom that do not participate in forming any bonds.
OF2 Hybridization
Oxygen atom shares two electrons with both Fluorine atoms, which means there are two bonding pairs of electrons in the Lewis structure of OF2 along with two nonbonding pairs of electrons on the central atom. To share electrons with Fluorine atoms, the orbitals of Oxygen undergo hybridization to accommodate the electrons.
The electronic configuration of the Oxygen atom in its ground state is 1s2 2s2 2p4
The 2s and 2p orbitals of Oxygen atom are hybridized, which means there is a formation of four hybridized orbitals: 2s, 2px, 2py and 2pz. And such hybridization makes this molecule sp3 hybridized.
So, OF2 or Oxygen Difluoride has sp3 hybridization.
OF2 Bond Angle
The electrons in the molecule are always arranged in a way that minimizes the repulsion between the electrons. In OF2, the electrons are arranged that tetrahedrally. Hence it has a tetrahedral electron geometry. The bond angle of F-O-H is 109° 27′.
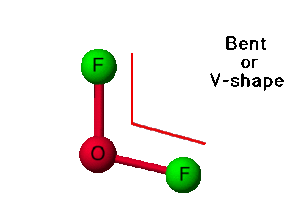
OF2 Molecular Geometry
Oxygen Difluoride has a similar arrangement like H2O. As only atoms are bonded with the central atom, it has a molecular geometry similar to AX2 that corresponds to linear geometry. Although the electron geometry of OF2 is tetrahedral, its molecular geometry is linear.
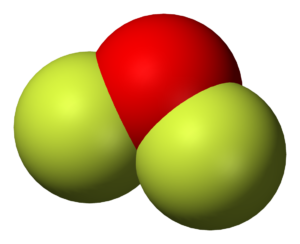
OF2 Shape
The shape of any molecule depends upon the arrangement of electrons and atoms in the molecule. Here both the Fluorine atoms are on the outside and share two valence electrons of the Oxygen atom. But as there are two lone pairs of electrons on the Oxygen atom, there will be repulsion between these two pairs, which causes a bend in the shape of its molecule. Even Though the molecule is linear, OF2 will have a bent shape.
Concluding Remarks
Oxygen Difluoride is a diatomic molecule made up of Oxygen and Fluorine atoms. To summarize this blog, we can say that:
- In the Lewis Structure of OF2, both Fluorine atoms share a single bond with the Oxygen.
- The central oxygen atom has two lone pairs of electrons, and the bond angle of F-O-F is 109° 27′.
- It has a linear molecular geometry and sp3 hybridization.
- OF2 has a bent shape and a tetrahedral electron geometry.