Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen. This protein is known to combine with Oxygen and deliver it to all the tissues in the body. But as this gas binds with hemoglobin, it doesn’t deliver oxygen to the tissues. This can convert hemoglobin to carboxyhemoglobin, which can lead to seizures, coma, and even fatality.
Although one can’t detect this gas as it has no odor, one should know that places without ventilation, closed cars, trucks, etc have a high concentration of Carbon Monoxide that can lead to Carbon Monoxide poisoning.
Here in this blog, we will look at the Lewis Structure of CO to understand other properties of this molecule.
| Name of molecule | Carbon Monoxide ( CO) |
| No of Valence Electrons in the molecule | 10 |
| Hybridization of CO | sp hybridization |
| Bond Angles | 180 degrees |
| Molecular Geometry of CO | Linear |
Contents
CO Valence Electrons
To draw the Lewis structure of CO, it is vital to know the total number of valence electrons of the molecule. The molecule has one Carbon atom and one oxygen atom. We will first find out the valence electrons for both these atoms and then add both these valence electrons to get the total valence electrons of CO.
Valence electrons of Carbon: 4
Valence electrons of Oxygen: 6
Total number of valence electrons: Valence electrons of Carbon + Valence electrons of Oxygen
: 4 + 6
: 10 valence electrons
Thus Carbon Monoxide has ten valence electrons. Now that we know the total number of valence electrons we can construct the CO Lewis structure.
CO Lewis Structure
The Lewis structure of Carbon Monoxide is relatively easy to understand, given there are only two atoms in this molecule. Lewis dot structure of any molecule helps to know the arrangement of valence electrons around its individual atom in the molecule. The electrons that do not form any bonds are called nonbonding pairs of electrons or lone pairs of electrons. The bonds in the molecule are shown by drawing lines.
Here there are only two atoms; let’s place the atoms besides each other like this.
C O
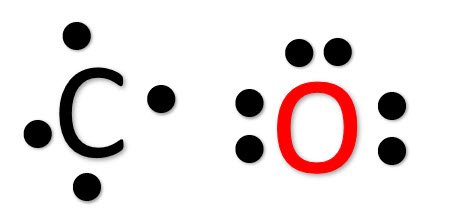
Now keep the valence electrons of both Carbon and Oxygen atoms around it like this.
You might notice Carbon has only four valence electrons and needs four more to complete its octet. In contrast, Oxygen has six valence electrons and only needs two more electrons to complete the octet.
In such molecules, atoms share electrons so that they can complete their octet. Both Carbon and Oxygen atoms will share one electron each to form a single bond. This leaves us with eight more valence electrons as two are used up.
But as you might notice, this bond is not enough for completing the octets of either of these atoms. Let us place more bonds to check if we can fill up the octets of both Carbon and Oxygen atoms.
Construct two more bonds between Oxygen and Carbon atom, which will use up four electrons more. Now we are left with four valence electrons only. To complete the octets of both these atoms, let’s place two valence electrons on both the atoms.
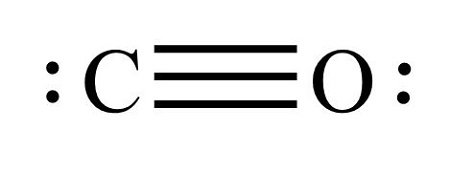
Here in Lewis structure of CO, you can see that Carbon shares three valence electrons of Oxygen after forming triple bonds and has one lone pair; its octet is complete, having eight electrons in its octet. Similarly, Oxygen has one lone pair of electrons, shares three electrons with Carbon, which completes its octet.
CO Hybridization
One can easily determine the hybridization of a given molecule if the molecule’s steric number is known. Here we will look at the steric numbers for both Carbon and Oxygen atoms.
Steric Number: Number of lone pairs attached to the atom + number of atoms attached
For Carbon, we have one lone pair of electrons and one atom (Oxygen) attached to it.
Hence Steric Number of Carbon will be: 1+1 = 2
Molecules having steric number 2 have sp hybridization. This will also hold true for Oxygen. Thus, the hybridization of both Carbon and Oxygen atoms in the Carbon Monoxide molecule is sp.
CO Molecular Geometry
Carbon Monoxide is a diatomic molecule with a triple bond between C and O and one lone pair of electrons on each atom. And since it only has two atoms, it has a linear molecular geometry. Carbon and Oxygen forms one sigma bond and two pi bonds.

Concluding Remarks
To conclude this article, we can say that:
Carbon Monoxide is a diatomic molecule having ten valence electrons.
Carbon and Oxygen atoms form triple bonds to complete their octets.
Both these atoms have one lone pair of electrons and sp hybridization.
Carbon Monoxide has a linear molecular geometry.