Someone who has just started with their chemistry lessons or needs a quick revision on what are the differences between Pyranose and Furanose; first, it is essential to know what these molecules are. Many biological molecules help in various mechanisms of our body and one such type of molecule are carbohydrates or also known as saccharides. Carbohydrates are the vital elements that help in cell signaling, supporting other processes and more. Another key feature of the carbohydrates is to store energy in the cells, from where we humans get our energy supply too. The term “sugar” is often used in the place of carbohydrates because of the structure of some molecules.
Pyranose vs Furanose
The definition of the term sugar is that it is a molecule having polyhydroxylated aldehydes or ketones except for the carbonyl carbon. When these sugar molecules come together and form a chain-like structure, it is called polysaccharides. So now that we have brushed the basics, let us know how pyranose differs from furnose. By now, you might be aware of glucose, a six-membered ring structure that is one of the most commonly known sugars. Well, there are other types of sugars as well such as Galactose, Mannose, Pyranose, Furnose and more. A lot of people tend to get confused between Pyranose and Furanose; hence we will go through their differences and features one by one.
The formation of cyclic forms of sugars

The sugar molecules can either exist in linear form or cyclic structures, depending upon their chemical properties. Glucose molecule can exist in linear or cyclic forms, but it quickly takes up the ring-like cyclic structure when it comes in contact with water. The reaction between the hydroxyl group of the water and ketone or aldehydes in the sugar molecule results in the formation of such cyclic saccharides. Depending upon the reaction and presence of OH and CHO groups on the carbon atoms in the molecule, different types of sugar or monosaccharides are formed.
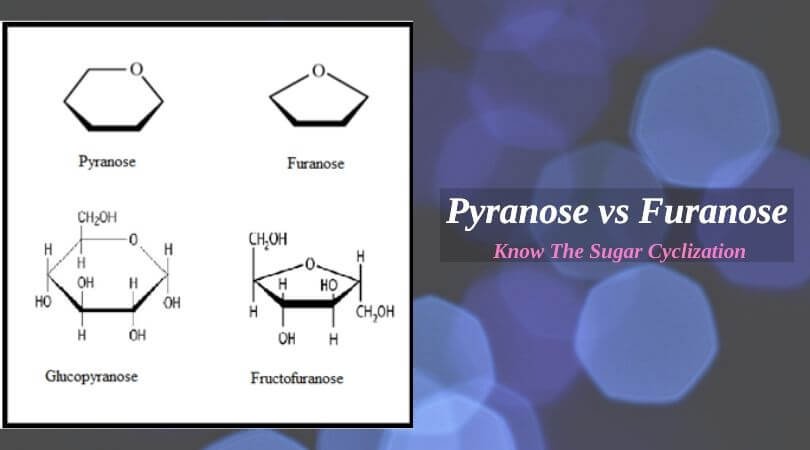
Both these monosaccharides pyranose and furanose exist in cyclic structure but differ in the structure formation. There is an anomeric carbon that acts as a chiral center for the cyclization of such molecules. Pyranose and furanose are the structural isomers; the initial one has a structure like a pyran molecule, while the latter one has a structural resemblance to furan molecules and that is how they have derived these names.
Pyranose

This monosaccharide has a six-membered ring that consists of five carbon atoms and one oxygen atom. There are no double bonds in the structure of pyranose and its ring structure is also known as Tetrahydropyran. As pyranose is an alpha (⍺) isomer of the d-glucose molecule, it is also termed as ⍺- D-(+)-Glucopyranose. The ring in pyranose is formed due to the reaction of the hydroxyl group (OH) on the fifth carbon (C5) of the sugar with the aldehyde group at C1.
Furanose

Furanose is also found from the mixture of sugars during the purification of glucose along with pyranose, but instead of a six-membered ring, this molecule has a five-membered ring consisting of four carbon atoms along with one oxygen atom. It is similar to pyranose as there are no double bonds in its cyclic structure too. If there is a reaction between the hydroxyl group on C4 and the aldehyde, the ring of furan is formed, which leads to the formation of the furanose molecule. The furanose ring can have either alpha or beta configuration depending upon the direction of an anomeric hydroxyl group.
Concluding Remarks
Pyranose and furanose both sugars are found in the aqueous solution of the saccharides, but they differ in the structure. This chemical difference also makes up for the differences in their physical properties such as boiling point, melting point, etc. The structure of pyranose is an isomer of Glucose and Furanose if that of fructose. But as the furanose has a five-membered ring, it is unstable as compared to the six-membered ring structure of pyranose.