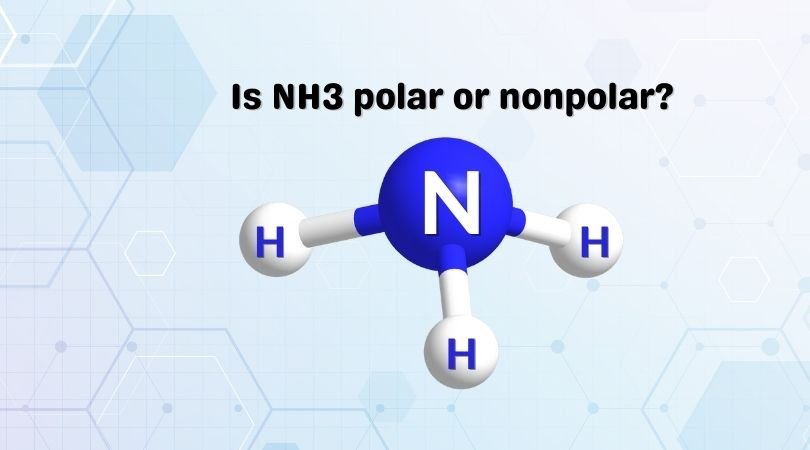
Ammonia or Nitrogen Trihydride is a pungent-smelling molecule that exists in a gaseous state at room temperature. A lot of students get confused when it comes to answering the question regarding the polarity of this molecule. In this blog post, we are going to find out if NH3 is polar or nonpolar.
NH3 Polarity
To know the polarity and other properties of any molecule, it is vital first to understand its Lewis structure. We have previously shared a detailed blog on the NH3 Lewis structure that you can check out for a quick revision of its lewis dot structure.
Ammonia has three single covalent bonds formed between the Nitrogen atom and the Hydrogen atoms, along with one pair of nonbonding electrons on the nitrogen atom. As the central atom forms bonds with three other atoms, it forms the trigonal pyramidal shape by sharing electrons. However, the bond angle decreases from 109.5 to 107 degrees due to the bonding pair of electrons’ repulsive forces.
So if you now closely look at the molecule, it has an asymmetrical shape which means it is not polar. As the electrons are arranged asymmetrically, there is a net dipole moment in this molecule. We now need to find the direction of the dipole moment.

Dipole moment in NH3
The vectors are directed towards the most electronegative atom, and it helps us to know the direction of the dipole moment in the molecule. In Ammonia, Nitrogen has an electronegativity of 3.04, whereas Hydrogen has the electronegativity of 2.2. Here as the Nitrogen atom is more electronegative than the Hydrogen atom, the dipole moment’s direction will be from the hydrogen atom towards the Nitrogen atom.
Only looking at the electronegativities cannot answer your question, consider all the dipole moments in order to get the net dipole moment of the molecule and its polarity. As NH3 is an asymmetrical molecule, the dipole moments are not canceled; hence there is a net dipole moment in the molecule, making Ammonia a polar molecule.
Also, as the difference between the electronegativities is relatively high, the N-H bonds are considered covalent polar bonds. This huge difference between the electronegativities leads to the unequal or asymmetrical distribution of the electric charges in the molecule.
Is NH3 polar or nonpolar?
Ammonia or NH3 is a polar molecule as there is a large difference of electronegativities between Nitrogen and Hydrogen along with the asymmetric shape of the molecule. The uneven dispersion of electric charges in the molecule makes it a polar molecule.