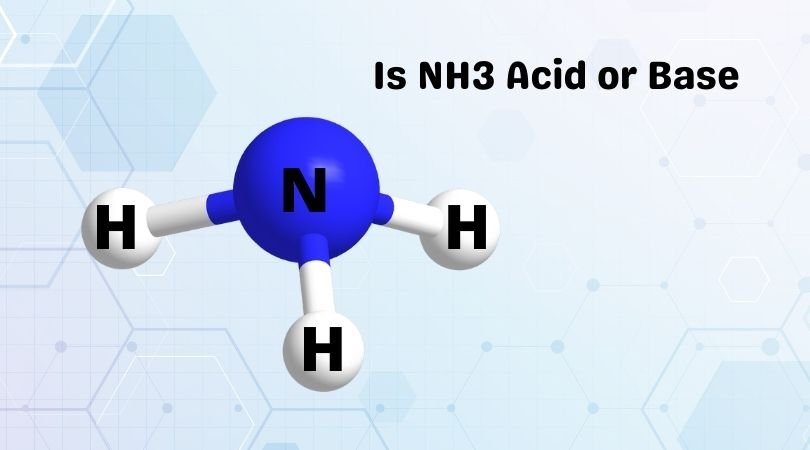
Ammonia or NH3 is one of the interesting compounds to study about. It has a pungent smell and a colorless, non-flammable gas. It is made up of one nitrogen atom and three hydrogen atoms. The distribution of electrons in Ammonia is asymmetric, which is why it is also a polar molecule. It has a trigonal bipyramidal molecular geometry and one of the known components in agriculture as it is widely used as a fertilizer. Ammonia is also used in manufacturing dyes, pesticides, and other such chemicals.
It is vital to know whether this molecule is a base or acid to understand its chemical properties further. Generally, a molecule is classified as a base or acid by looking at its pH. Ammonia is different from other molecules, and hence one-word answer for this question wouldn’t suffice. Keep reading this blog post to find out if NH3 is an acid or base.
Is NH3 acidic or basic?
Ammonia has a pH 11, which makes it a weak base. Generally, the compounds having a pH between 7 to 14 is base. Here although Ammonia is a weak base, it is amphoteric as it can act as an acid as well as a base depending upon the conditions in which the experiments are conducted.
Ammonia can act as a weak base under a suitable condition and accepts H+, which results in forming NH4+, a conjugate acid. Whereas under certain conditions, NH3 can act as a weak acid and donate one H+ that forms its conjugate base NH2+. According to Bronsted-Lowry theory, any compound that accepts a proton is considered as Bronsted-Lowry base. Here as Ammonia has a lone pair of electrons and accepts a proton, it is considered as a Bronsted-Lowry Base.
So although NH3 is technically a weak base, it can act as a base and acid depending upon the situations and the molecules it is reacting with.