If you have been reading about general chemistry lately and know a bit about it, you might be aware that each reaction that takes place can be either classified as an endothermic reaction or an exothermic reaction. So any reaction you see in your household or surroundings can be classified accordingly. In this blog post, we will find out if boiling water is an exothermic or endothermic reaction.
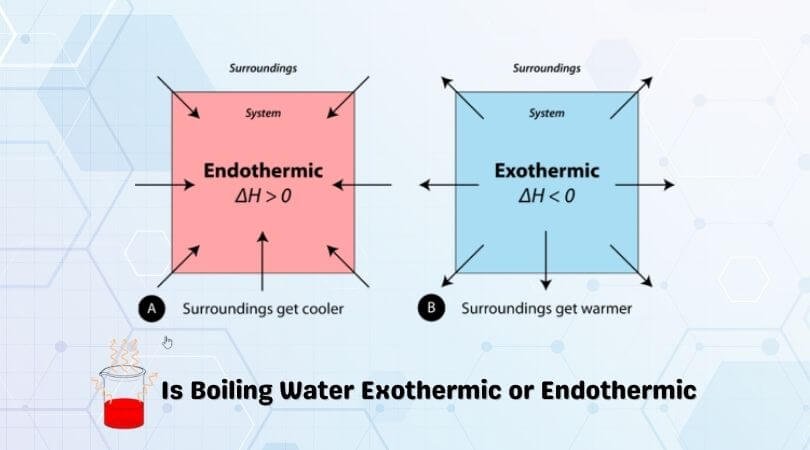
First, let’s take a look at what “endothermic” means. The word “endo-” means within or into, while “therm” means heat or energy. So an endothermic process is one in which heat (or energy) is going into the system.
“Exothermic” reactions are the ones that release the energy into the surroundings when the chemical reaction takes place. So basically, any reaction that releases energy is classified as an exothermic reaction.
So now, if you look at the boiling water reaction, it does not spontaneously boil at room temperature. Instead, heat has to be supplied to the water molecules to increase their potential energy. This applied heat causes the water molecules to move further away from each other without causing any increase in overall temperature. As the heat is given to the reaction, it means that it is an endothermic reaction. You can also see it the other way; the water wouldn’t start boiling unless you provide the system with heat or energy. Therefore, boiling water is an endothermic process.
The applied heat helps break the intermolecular bonds between the water molecules, which spreads in the molecules further apart. The water turns completely to steam (water vapor) when all the intermolecular bonds are broken.
The physical process explained above (water turning into water vapor) is called a phase change. This is because once the intermolecular bonds are broken, any extra heat increases the system’s kinetic energy, and the molecules of the water vapor move faster as the temperature increases.
Supplying heat to the water helps it transition from liquid to gas, and hence there is a change in the energy of the water that is boiled. When the state of the subject changes from liquid to gaseous, that process requires either input of energy or output of energy. This energy is often in the form of thermal energy or heat. A lot of chemical reactions require such input of energy and are referred to as endothermic reaction.
Now, you might wonder if melting ice was an endothermic or an exothermic reaction. Of course, it is an exothermic reaction, as there is no heat supplied to melt the ice; instead, the energy is released. And such a reaction is referred to as an exothermic reaction.
Concluding Remarks
As one needs to supply heat energy to boil water, this chemical reaction is considered an endothermic reaction. Here the heat energy is provided, which breaks the bond of molecules/atoms in the liquid state. Hence, boiling water is an endothermic reaction.