Hydrogen Peroxide or H2O2 is widely used as an oxidizing agent and as an antiseptic. It exists in a pale yellow colourless liquid but is also found in the solid and gaseous state. Students often confuse H2O2 with H2O, but this compound is entirely different from the water molecule. It has a bitter taste and is quite unstable when exposed to light.
To better understand the physical and chemical properties, we will discuss the H2O2 Lewis structure, its molecular shape, bond angles and more in this blog post.
| Name of molecule | Hydrogen Peroxide ( H2O2) |
| No of Valence Electrons in the molecule | 14 |
| Hybridization of H2O2 | sp3 hybridization |
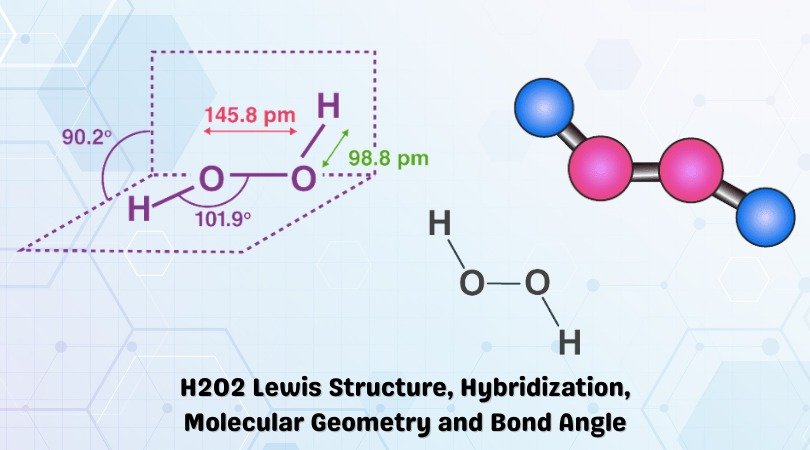
| Bond Angles | 101.9° (S) and 94.8° |
| Molecular Geometry of H2O2 | Trigonal pyramidal |
Contents
H2O2 Valence electrons
Hydrogen Peroxide comprises two Hydrogen atoms and two Oxygen atoms; hence we will need to know the valence electrons for both atoms to get the total valence electrons for H2O2.
Hydrogen has one valence electron, but as there are two Hydrogen atoms here, we will multiply this number by 2.
Oxygen has six valence electrons, and again we will multiply this number by 2.
H2O2 total valence electrons – Valence electrons of Hydrogen + Valence electrons of Oxygen
= 1(2) + 6(2)
= 2+ 12
= 14 valence electrons
Hydrogen Peroxide or H2O2 has 14 valence electrons.
H2O2 Lewis Structure
The Lewis dot structure for any molecule or compound helps determine the arrangement of atoms in the molecule, bonds formed, and lone pairs of electrons. For determining the Lewis structure, remember the following points:
- Each element follows an octet rule, in which it tries to attain a stable structure by having eight valence electrons in its outer shell. ( There are some exceptions to this rule, e.g., Hydrogen)
- Always know the total number of valence electrons for the molecule; here, H2O2 has 14 valence electrons.
- Hydrogen atoms never take a central position in the Lewis structure.
So now, for H2O2 Lewis Structure, we first put the central atoms and show their bond formation with other atoms.
As there are two Oxygen atoms here, both of these oxygen atoms take the central position and share two valence electrons to form a bond.
Now place two Hydrogen atoms next to these Oxygen atoms.
Each Hydrogen atom only requires one more valence electron to complete its octet, and hence it forms a bond with the neighbouring Oxygen atom. As a result, four more valence electrons are used in total as both these Hydrogen atoms form a single bond with oxygen atoms.
We used up six valence electrons out of 16 ( there are three single bonds in these molecules. Arrange the remaining eight electrons around the Oxygen atom. Each Oxygen atom will have four valence electrons or two pairs of electrons that do not participate in any bond formation. These pairs of electrons are called non-bonding or lone pairs of electrons.

As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. There are three single bonds formed in these molecules, and hence there are 3 bonding pairs and four lone pairs of electrons in H2O2.
H2O2 Hybridization
Determining hybridization for any molecule becomes easy and quick once you know the central atom, the atoms forming bonds with it and the number of lone pairs of electrons. Here both Oxygen atoms are forming bonds, and hence these two Oxygen atoms undergo hybridization.
If you consider one Oxygen atom, for now, it forms a bond with the Hydrogen atom, neighbouring oxygen atom and has two lone pairs. In short, it needs to form four hybrid orbitals. You can also find the steric number for finding the hybridization; this way, one can easily get the hybridization of any molecule.
The steric number of H2O2 also corresponds to 4, which means it is sp3 hybridized. There is one s orbital and three p hybrid orbitals to form to accommodate the lone pairs as well as the bonding pairs of electrons.
H2O2 Molecular Geometry
VSEPR theory helps us determine the molecular geometry of any given molecule. Here two Oxygen atoms are forming bonds with Hydrogen atoms. However, there are two lone pairs of electrons on each Oxygen atom. And as per VSEPR theory, these lone pairs try to repel each other, which distorts the molecule’s shape.
The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral.
One can also use the AXN Notation method to determine the geometry of the molecules. For H2O2, the notation is AX2N2 which corresponds to tetrahedral geometry in the table given below.

H2O2 Bond Angle
Generally, the molecules having tetrahedral geometry and AX2N2 notation, predicted bond angle for H2O2 is 104.5°. However, there are two lone pairs of electrons in the molecule that tweak this bond angle a bit, and as a result, H2O2 has a bond angle of 101.9° in its solid-state and a bond angle of 94.8° in a gaseous state.
The bond angles differ when the state of the molecule changes as the intermolecular forces between the electronegative Oxygen atom decreases, and as a result, the bond angle decreases. Hence the bond angles for H2O2 are 101.9° (S) and 94.8° (G).

H2O2 Shape
Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. As the molecule is not linear and has a tetrahedral electron geometry, the molecular shape of Hydrogen Peroxide is bent.
Hence, H2O2 is a bent molecule.
Concluding Remarks
To summarize this blog post on H2O2, we can conclude the following:
- Hydrogen Peroxide has two atoms of Hydrogen and two Oxygen atoms.
- There are a total of 14 valence electrons for H2O2.
- In the Lewis Structure of H2O2, there are three single bonds formed having two H-O bonds and one O-O bond.
- There are two lone pairs of electrons on each Oxygen atom; thus, there are four lone pairs of electrons for H2O2.
- As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization.
- The bond angle for H2O2 in its gas phase is 94.8°and has a bond angle of 101.9°.
- It has tetrahedral electron geometry and a bent molecular shape.