One of the first molecules used for studying and understanding molecular geometry and the basics of the Lewis structure is CH2O. This molecule is also referred to as Formaldehyde and has a simple molecular geometry as compared to other complex molecules. Formaldehyde, also termed Methanal is a naturally occurring organic compound having a chemical formula of CH2O. It is a pungent-smelling gas and a simple aldehyde with R-CHO’s empirical formula. R represents the molecule, and CHO is used to represent the functional group of aldehyde.
To know the physical and chemical properties of CH2O, it is vital to know the Lewis structure, valence electrons, and hybridization of the molecule. Let us have a look at all these properties one-by-one.
Contents
CH2O valence electrons
As one can make out from the chemical formula itself, one molecule of Formaldehyde or Methanal has one Carbon atom, two Hydrogen atoms, and one Oxygen atom. It has the chemical structure of H-CHO, where the Hydrogen atom is attached to the aldehyde functional group.

Each atom has different valence electrons in its outer shell. And the cumulative Valence electrons of any given compound are essential for know its Lewis structure. As per the octet rule, a compound should have two or eight electrons in its outer shell to attain a chemical structure similar to the inert elements, where they can be completely stable and do not react with any other compound.
To find out the valence electrons of CH2O, we have to first look at the valence electrons of all the atoms individually:
- The carbon atom has a valency of four as it has four electrons in its outer shell.
- The oxygen atom has a valency of two as it has six electrons in its outer shell.
- A hydrogen atom has a valency of one as it only one electron in its outer shell.
Total valence electron of CH2O= Valence electrons of Carbon + Valence electrons of Oxygen + Valence electrons of Hydrogen
= 4+6+2*1
= 12 valence electrons of CH2O
Thus, CH2O has a total of twelve valence electrons that can help in drawing its Lewis structure.
Formaldehyde Lewis structure
Lewis structure is a pictorial representation of the atoms in the molecules, their bonds, and lone pairs of electrons. The bonds are represented by drawing lines, whereas the electrons are represented by dots. Thus it is also called dot and line structure.
Here for Methanal or Formaldehyde, we have 12 valence electrons. As you know by now, the Hydrogen atom is always on the terminal side. Between the rest two atoms, Carbon is the least electronegative atom, and hence it will be in the center. So put the Carbon atom in the center, two Hydrogen atoms on the terminals, and place the Oxygen atom above the Carbon atom.
As Carbon has a valency of four, it needs to share or gain four electrons with other atoms to complete its octet. Here there are two Hydrogen atoms with one electron in their outer shell; the central atom shares these two electrons and brings the valency of Carbon to six. On the other side, the Hydrogen atom now has two electrons in its outer shell, which suffices the octet rule. Carbon atom forms single bonds with these two Hydrogen atoms that are on the terminals of the molecule.
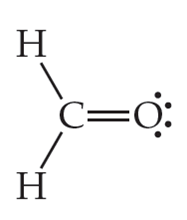
Next, the Carbon atom forms bonds with Oxygen, where it shares two valence electrons of the atom to complete its octet. Here, both the atoms share two electrons, and hence there is a double bond between Carbon and Oxygen atoms to complete the central atom’s octet. This double bond is represented by drawing two parallel lines in the Lewis Structure.
After these bond formations, the Oxygen atom is left with four valence electrons, which means it has two lone pairs of electrons.
Thus in the Lewis structure of CH2O, the central Carbon atom forms two single bonds with two Hydrogen atoms and one double bond with an Oxygen atom. Here the octets of both Carbon and Hydrogen are completed, and only Oxygen has two lone pairs of electrons.
CH2O Lewis structure resonance
CH2O has resonance structures, which means that the compound’s single Lewis structure is unable to explain all the bonding in the molecule due to the presence of partial charges in the compound. Here there are partial charges on Oxygen, which results in delocalization of an electron or charge. This results in a changed arrangement of the electrons but with the same formula. There is no change in the chemical formula or the compound’s properties, but the charges on electrons shift.
In its resonance structure, the Lewis structure of CH2O has all single bonds instead of a double bond between Oxygen and Carbon. Most compounds with partial charge distribution have resonance structures that only differ in the electrons’ arrangement in the molecule.
CH2O Hybridization
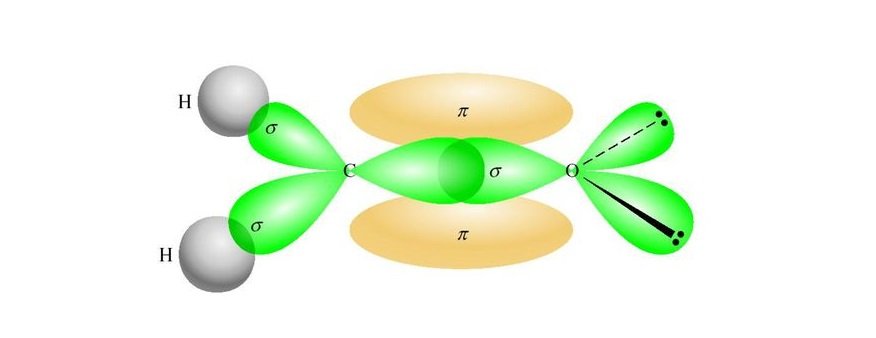
Once we know the Lewis dot structure of Formaldehyde, we can easily find out its hybridization and molecular geometry. Using the VSEPR theory, it can be seen that CH2O represents the chemical formula of AX3. And as per the VSEPR rule, compounds with AX3 have sp2 hybridization. As only one s-orbital and two p-orbitals of the central Carbon atom are hybridized, it leads to the sp2 hybridization.
Concluding Remarks
Formaldehyde, more commonly known as Methanal, is the simplest aldehyde having one Carbon atom, two Hydrogen atoms, and one oxygen atom. It has a total of 12 valence electrons. The Lewis Structure of CH2O has two single bonds between the central carbon atom and two hydrogen atoms on the terminals and a double bond with the Oxygen atom. There are no lone pair of electrons on the central Carbon atom, but the Oxygen atom has two lone electron pairs. The molecule has sp2 hybridization, which can help in knowing the molecular geometry and polarity of CH2O