The chemical formula CF4 represents Carbon Tetrafluoride. It is also known as a Tetrafluoromethane (IUPAC name) and R-14 (owing to its use as a refrigerant).
CF4 is the simplest perfluorocarbon (Hydrocarbons in which C-F bonds have replaced all the C-H bonds). The compound is very stable and does not react with acids or hydroxides.
The C-F bond is one of the strongest chemical bonds and has bonding energy of 515 kJ⋅mol−1. This makes it one of the strongest bonds in organic chemistry. This strength is a result of Fluorine’s electronegativity, which shortens and strengthens the C-F bond.
Carbon Tetrafluoride is colorless and highly dense. It reacts explosively with alkali metals such as Sodium. Thermal decomposition of the compound produces Carbon Monoxide and Carbonyl Fluoride (toxic gases).
It is produced industrially when Hydrogen Fluoride reacts with dichlorodifluoromethane or chlorotrifluoromethane. Other methods of preparation include Fluorination and electrolysis. In a laboratory, CF4 can be prepared by the reaction of silicon carbide with Fluorine.
It finds use as a low-temperature refrigerant and in electronics manufacturing.
It is a greenhouse gas, but unlike CFCs, it doesn’t deplete the Ozone layer due to the strong Carbon-Fluorine bond.
CF4 has the following properties:
| Name of the molecule | Carbon Tetrafluoride (CF4) |
| No. of valence electrons | 4 + (7 x 4) = 32 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 109.5° |
| Molecular Geometry of CF4 | Tetrahedral Molecular Geometry |
Contents
CF4 Valence Electrons
Valence electrons are those electrons that are available for exchanges and bond formation. They are present in the atom’s outermost shell, where the force of attraction from the nucleus is relatively less. This, in turn, makes these electrons readily available upon excitation.
Carbon Tetrafluoride comprises four Fluorine atoms and a single Carbon atom.
Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, a single carbon atom contributes: 4 x 1 = 4 Valence Electrons.
Fluorine is in group 17 of the periodic table with the electronic configuration [He] 2s22p5. Therefore, the four Fluorine atoms present contribute: 7 x 4 = 28 Valence Electrons.
Therefore, the total number of valence electrons in CF4 is given by:
4[C] + 28[F] = 32 Valence Electrons
As a result there are 32 valence electrons for CF4.
CF4 Lewis Structure
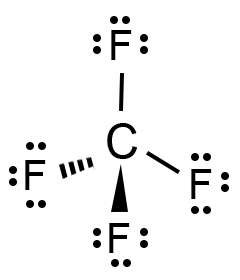
Carbon acts as the central atom in the structure. This arrangement lets it form covalent bonds with the surrounding Fluorine atoms. The Fluorine atoms in CF4 are then placed around the central Carbon atom.
We then begin drawing up the Lewis structure by forming C-F bonds using the available valence electrons. From the figure below, it is seen that the central Carbon atom forms four covalent bonds.

We then proceed to fulfill octets for the outermost atoms, i.e., the four Fluorine atoms. As shown in the figure, the remaining valence electrons surround each of the Fluorine atoms, fulfilling their octets.
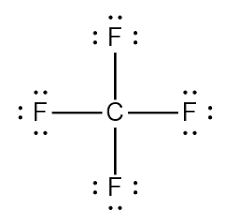
There is no need to check for formal charges as the octet rule is fulfilled for all of the atoms as shown. The Lewis Structure is stable due to all of the outermost shells being filled for each element.
CF4 Hybridization
The Carbon Tetrafluoride molecule comprises four Fluorine atoms, all pulled together by the central Carbon atom.
To determine the hybridization, we take a quick look at the Lewis structure of the CF4 molecule shown above. There are four covalent bonds present between Carbon and Fluorine. This structure gives rise to four electron domains.
The number of electron domains is an easy way to determine the hybridization of a molecule. This is especially true for non-polymeric arrangements.
To know more about the hybridization of CF4 in detail, we look into the nature of the C-F bond. Sigma bonds are formed between Carbon and Fluorine. Pi-bonds are absent, making the structure remarkably stable.
As such, the hybridization of the central Carbon atom is sp3.
CF4 Bond Angles
According to the VSEPR theory, the Fluorine atoms all repel each other forming a tetrahedral shape. As such, the bond angle of CF4 is 109.5°.
CF4 Molecular Geometry and Shape
Going back to the Lewis Structure shown above, we observe that the Fluorine atoms are surrounded by electrons. These electrons repel each other. Therefore, in accordance with the VSEPR theory, the Fluorine atoms push as far away from each other as possible.

This results in a Tetrahedral molecular geometry, as shown. This can also be determined by using the steric number or the A-X-N method.
‘A’ represents the central atom. The value of ‘A’ here is 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, there are four Fluorine atoms bonded to the central Carbon atom.
Therefore, X =4.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 0.
Therefore, that would give us AX4 for the CF4 molecule. We can ignore ‘N’ since there are no lone pairs. From the A-X-N table below, we can determine the molecular geometry.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
Therefore, the molecular geometry of CF4 is Tetrahedral. Since there are no lone pairs, the electron geometry is Tetrahedral as well.
Concluding Remarks
Let’s quickly summarize the salient features of Carbon Tetrafluoride
- CF4 comprises a Carbon atom surrounded by four Fluorine atoms.
- In its most stable state, the Carbon atom forms covalent atoms with the Fluorine atoms. There are no lone pairs.
- The hybridization of the CF4 is given by sp3.
- CF4 has a Tetrahedral molecular structure and shape with bond angles of 109.5°.