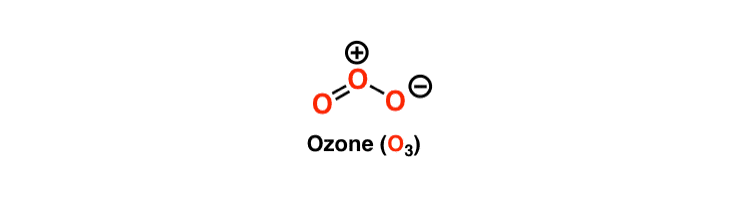
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations. To understand the hybridization, polarity and molecular geometry of the Ozone molecule it is crucial to know the Lewis structure of the same.
| Name of molecule | Ozone (O3) |
| No of Valence Electrons in the molecule | 18 |
| Hybridization of O3 | sp2 hybridization |
| Bond Angles | 116 degrees |
| Molecular Geometry of O3 | Bent |
Now, a lot of people ask why it is necessary to know the Lewis structure of any given molecule or compound. The answer to this question is simple; this structure helps in understanding the basic structure, electrons that take part in bond formation along with the charges on a given atom.
Lewis structure is based on the octet rule. The octet rule states that there should be eight electrons in the outer shell or orbit of the atom for the molecule to be stable. Lewis structure helps to know the number of valence electrons in the molecule. Valence electrons are the electrons that participate in bond forming and nonbonding electrons pairs.
The electrons that take part in the bond formation are known as bonding pairs of electrons. These electrons are represented by drawing lines in the Lewis structure. Whereas the electrons that do not take part in the bond formation are non-bonding pairs of electrons. Dots around the central atom represent these electrons.
Contents
O3 Valence electrons
In Ozone or O3, there are six valence electrons for each molecule of Oxygen.
Here as there are three oxygen molecules, the total number of valence electrons is 6*3= 18.
Thus there are a total of 18 valence electrons available for Ozone molecule.
O3 Lewis Structure
As the octet rule applies in this structure, the central atom is the first one that should have eight electrons in its outer shell. So one molecule of the Oxygen is in the centre with the other two are on the opposite sides.
The central atom has one lone pair of electrons and is stable due to the eight electrons in its outermost orbit. To satisfy the octet rule, a central atom needs to form a double bond on either of its sides with an Oxygen molecule and another single bond. As both the molecules of Oxygen have the same electronegativity and structure, the double bond keeps on shifting from both the molecules.

Resonance structures of O3
However, the structure of Ozone is unique as the central atom has one double bond and one single bond with its neighbouring oxygen molecules. These bonds keep interchanging their places, and hence the ozone has resonant lewis structure. The resonance means the constant interchanging of the bonds between the three molecules in Ozone. The central atom in the Lewis structure will have a charge of +1 and the atom forming a single bond will have -1 charge.
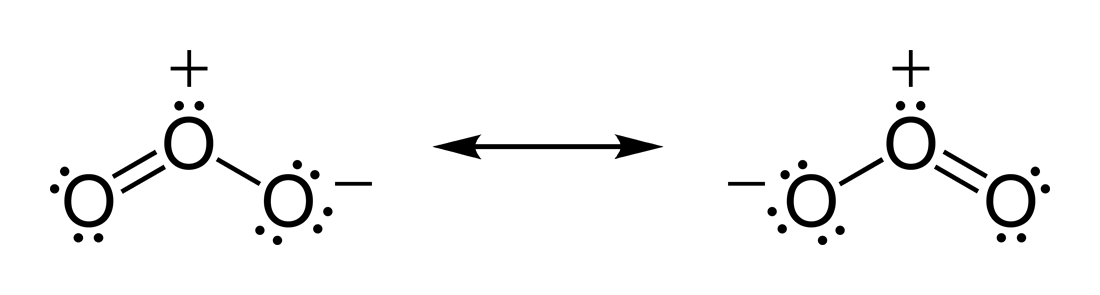
O3 Hybridization
Hybridization in chemistry means the hybridising of two or more atomic levels of the same or different energies to combine and give a new orbital. Once we know the Lewis structure of a molecule, it is easy to know the hybridization of it. As Ozone has one central Oxygen atom having eight electrons in its outermost shell, the hybridization for the central atom will be sp2. There are two electrons in the 2s orbital, whereas 6 electrons in both the 2p orbitals out of three 2p orbitals. As there are electrons in one s orbital, and two p orbitals, the hybridization of the central oxygen atom becomes sp2.
Other two oxygen atoms also have hybridization. One will have sp2 hybridization, whereas, the other will have sp3 hybridization as there is one lone pair of electrons that creates resonance in the structure of Ozone. As we always consider the hybridization of the central atom as the final hybridization, Ozone has sp2 hybridization.
O3 Molecular Geometry
As the hybridization of the molecule determines its shape, we can now know the molecular geometry of Ozone. Ozone has sp2 hybridization means that it should have a trigonal planar shape. But as the structure of Ozone has resonance and one lone pair of electrons, the angle between the molecules is less than 120 degrees.
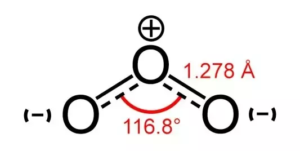
O3 Bond Angles
There is always a repulsive force between the bonding electrons which is lower than the repulsion between a lone pair and bonding electrons. In this case, as there is only one pair of lone electrons, there is a decrease in the angle from 120 to 116 degrees.
O3 Shape
This reduction in the angle from 120 to 116 degrees causes a bent in shape, which results in a distorted trigonal planar shape. Many times the shape of Ozone is also considered bent or planar due to its distortion.
O3 Polar or Nonpolar
The polarity of every molecule depends on its molecular geometry. Here, the Ozone molecule is bent due to its valence electrons. All three Oxygen molecules are not linear due to their sp2 hybridization. As the molecules are not in linear geometry their dipole interactions are not nullified, and as a result there is a net dipole in this molecule. Thus there is polarity in Ozone, and it can be said that Ozone is polar. This polarity is due to one lone pair of electrons on the central atom of Ozone.
Concluding Remarks
To put everything together, we can say that:
Ozone has 18 valence electrons out of which there is one lone pair of electrons.
It has sp2 hybridization, trigonal planar ( bent/ angular) geometry and is polar.


Thank you for the explanation, it sure does help!
That’s the aim, Luigi.