Carbon Tetrachloride is a colourless liquid with a little smell. It was generally used in fire extinguishers and refrigerators as a precursor for cleaning. The compound has a chemical formula of CCl4 and is now banned from use as it has some toxic properties that can harm the central nervous system of humans. Carbon Tetrachloride was first synthesized as a by-product in the synthesis of Chloroform. For understanding the physical and chemical properties of this organic compound, it is vital to know the Lewis structure, hybridization and much more. In this article, we will discuss all such properties to understand the structure of the compound.
Contents
Lewis Structure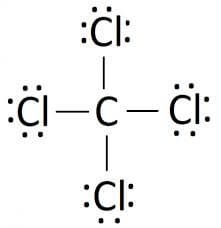
In chemistry, the basis of understanding any property of the compound depends on its lewis structure. G.N Lewis first proposed this theory in 1916 that helps in understanding the involvement of electrons informing the structure of the chemical. The electrons that participate in forming the bonds are known as the bonding pair of electrons. The ones that do not participate in it are known by the term non-bonding or lone pair of electrons. The bonding, as well as non-bonding electrons collectively, are called valence electrons.
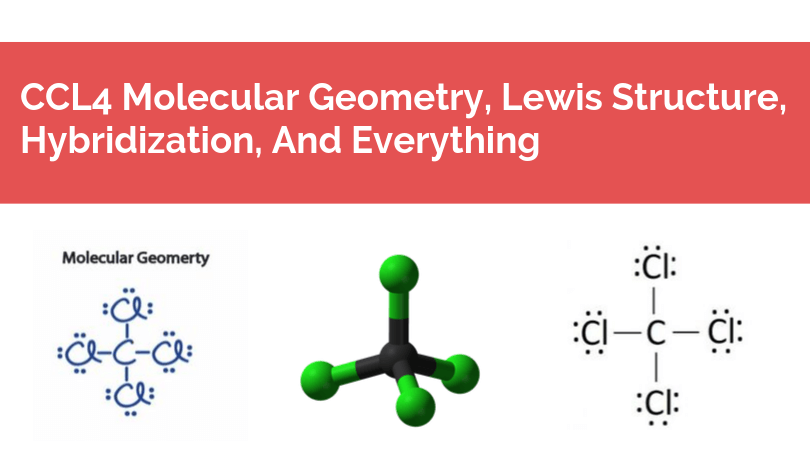
The Lewis structure is the pictorial representation of the valence electrons that participate in the bond formation as well as the ones that don’t. Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable.
For the Lewis structure of CCl4 first, let’s calculate the total valence electrons.
Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly.
= 4 + (4*7)
= 4 + 28
= 32 valence electrons
All four valence electrons of Carbon participate in the bond formation. Similarly, a single electron form each Chlorine atom participate in bond formation. Total 8 electrons make the bonds while others are non-bonding pairs of electrons.
=32-8
=24
So there are a total of 24 non-bonding or 12 lone pairs of electrons in CCl4. Four lines in the structure represent four bonds while dots around the Chlorine atom represent valence electrons. Each Chlorine atom has six valence electrons after the bonds are formed.
Hybridization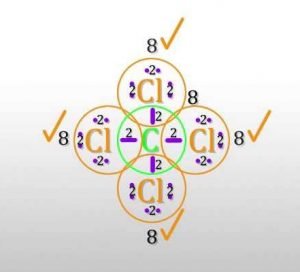
Hybridization is vital to understand the molecular geometry of the compound. When the two or more orbitals hybridize, the orbital is known as the hybrid orbitals. These orbitals are formed when there are bond formations in the compound.
For this compound, there are four covalent bonds between the central Carbon atom and four chlorine atoms. As all the valence electrons of Carbon are involved in bond formation, all orbitals of the atom participate in the formation of hybrid orbitals. In this one s orbital and three p orbitals of Carbon atom hybridize and form a sp3 hybridization. So the hybridization of Carbon Tetrachloride becomes sp3 as all the orbitals of the Carbon atom are hybridized.
Molecular Geometry

Once we know the Lewis structure and hybridization of the compound, it becomes easy to understand the molecular geometry of the compound. For this compound, the Carbon atom in the central position and rest all the Chlorine atoms are placed around it. As the central atom has four bonded pairs and sp3 hybridization, the shape of the molecule is tetrahedral.
Rest all the non-bonding electrons are spread out in the structure. There are repulsive forces between the lone pair of electrons. Due to this repulsion force, the lone pairs tend to go far from each other in the plane. The bond angle between these lone pairs of the electron is 109.8 degrees. Another way to know the molecular geometry is by using VSEPR theory which also states that the shape of this molecule is tetrahedral.
Polarity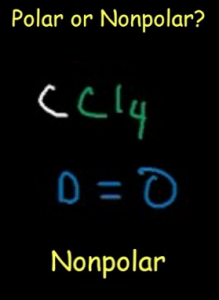
The polarity of any compound depends on its molecular geometry. When the bonding and non-bonding pairs are arranged in the plane, there is some dipole moment between them which makes the molecule polar. The arrangement of the lone pairs and the shape of CCl4 is such that the dipole moment of electron pairs get nullified. Due to this, there is no polarity observed in Carbon Tetrachloride. Thus CCl4 is nonpolar. This polarity property of the compound is due to the symmetric distribution of the non-bonding pairs of electrons in the plane.
Concluding Remarks
To summarize this article, it can be said that Carbon Tetrachloride has total 32 valence electrons out of which 8 electrons participate in bond formation. The rest 28 electrons are non-bonding electrons. Carbon completes its octet by forming bonds with four chlorine atoms. The hybridization of CCl4 is sp3 and has a tetrahedral shape. The bond angle is 109.8 degrees between the lone pairs of electrons and it is nonpolar.