Phosphoryl Oxychloride is a colorless liquid having a chemical formula of POCl3. It is also known as Phosphoryl chloride. The molecule is made up of one Phosphorus atom, one oxygen atom, and three chlorine atoms. POCl3 is widely used in phosphate esters production and as a dehydrating agent in the Bischler-Napieralski reaction. In this blog post, we will look at the Lewis structure, molecular geometry, and shape of the molecule.
| Name of molecule | Phosphoryl Oxychloride (POCl3) |
| No of Valence Electrons in the molecule | 32 |
| Hybridization of POCl3 | sp3 hybridization |
| Bond Angles | 109.8° |
| Molecular Geometry of POCl3 | Tetrahedral |
Contents
POCl3 Valence electrons
To determine the Lewis structure of any molecule, we first need to know the total number of valence electrons for the molecule. The electrons that are present in the outer shell are called valence electrons. These valence electrons participate in bond formation.
For finding out the total number of valence electrons for POCl3, we find the valence electrons of all the atoms.
Phosphorus has five valence electrons.
Oxygen has six valence electrons.
Chlorine has seven valence electrons, but as there are three chlorine atoms, we will multiply the number by 3.
Total number of valence electrons in POCl3 – Valence electrons of phosphorus + valence electrons of Oxygen + valence electrons of chlorine
= 5+6+7*3
= 32 valence electrons
Thus, POCl3 has 32 valence electrons.
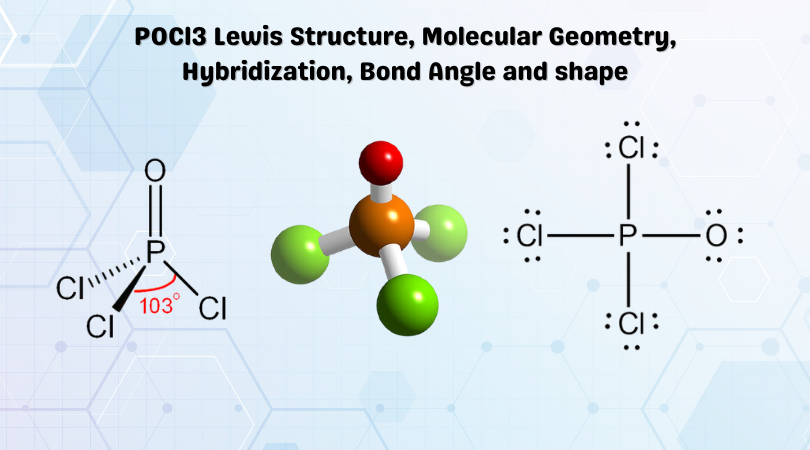
POCl3 Lewis Structure
The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone pairs of electrons. Here we will look at the Lewis structure of POCl3 to further determine its molecular geometry, bond angle, and shape.
Phosphorus atom will be placed in the center as it is the least electronegative out of all the atoms. Rest all chlorine and oxygen atoms will be placed around this central atom.
Chlorine atom needs one valence electron to complete its octet; hence it will share one valence electron of phosphorus. Thus there are three single bonds formed between Phosphorus and Chlorine atoms.
After forming bonds with Chlorine atoms, the Phosphorus atom is now left with two unshared valence electrons as it has shared three out of five electrons with chlorine. Oxygen requires two valence electrons to complete its octet.
After sharing three valence electrons with chlorine, the Phosphorus atom has eight valence electrons in its outer shell. But as it belongs to Group 15 on the Periodic table, it can share all its five valence electrons to attain a stable structure. In this molecule, phosphorus will share its remaining electrons with Oxygen by forming double bonds. As there are double bonds formed between Phosphorus and Oxygen atoms, all the atoms now have a complete octet.
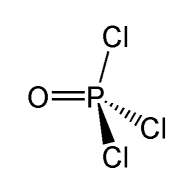
Thus, phosphorus forms three single bonds with Chlorine atoms and one double bond with Oxygen atom.

POCl3 Molecular Geometry
In Phosphorus Oxychloride, the central atom forms bonds with four atoms – three chlorine atoms and one oxygen atom. All the atoms are arranged as far as possible from each other to minimize the repulsive forces among the bonding pairs of electrons. According to VSEPR theory, the molecule adopts a geometry to keep these repulsive forces at a minimum. POCl3 has a tetrahedral molecular geometry as the central Phosphorus atom is forming bonds with four atoms.

POCl3 Hybridization
The hybridization of an atom in the molecule can be determined by looking at its steric number. For this molecule, we will look at the phosphorus atom’s hybridization as it is in the central position.
Steric number – Number of atoms attached + number of lone pairs
For phosphorus, the atom forms bonds with four atoms and has no lone pairs of electrons.
= 4+0
=4
As the steric number of the Phosphorus atom is 4, it has sp3 hybridization. The atom needs to have four hybrid orbitals to accommodate all the electron pairs. Hence, POCL3 has sp3 hybridization.
POCl3 Bond Angle
When we look at the AXN table to determine the molecular geometry and bond angles of the given molecule, we can see that the phosphorus forms bonds with four atoms and has no lone pairs of electrons. Thus it is similar to AX4, where A is the central atom and X is the number of atoms forming bonds with the central atom. And as it has a tetrahedral shape, it has bond angles of 109.8, although it might differ a bit due to the double bond in the molecule.

POCl3 Shape
The shape of the Phosphorus Oxychloride is tetrahedral. As all the four atoms are arranged in symmetry around the phosphorus atom, the shape is not distorted and is arranged in a tetrahedral shape.
Concluding Remarks
To summarize this article, we can say the following:
- Phosphorus Oxychloride consists of one Phosphorus atom, three chlorine atoms, and one oxygen atom.
- There are 32 valence electrons for POCl3 that participate in forming bonds.
- Phosphorus forms three single bonds with three chlorine atoms and a double bond with an Oxygen atom.
- Phosphorus has sp3 hybridization in this molecule.
- The bond angles are approximately 109.8°, and the molecular geometry of this molecule is tetrahedral.