The chemical formula AlCl3 represents the chemical compound Aluminum Chloride. Anhydrous Aluminum Chloride is white; however, the presence of Iron Chloride gives the compound a pale yellow color. AlCl3 exists in different states (solid, liquid, gaseous) depending upon temperature. It also possesses a strong odor of Hydrogen Chloride.

Anhydrous AlCl3 is hygroscopic and vigorously reacts with water to form an acidic aqueous solution[1].
AlCl3 + 3H2O → Al(OH)3 + 3 HCl
Al(H2O)6Cl3 → Al(OH)3 + 3 HCl + 3 H2O
The Cl– ligands are displaced by water molecules to form a hexahydrate- [Al(H2O)6]Cl3. This reaction cannot be reversed, and the anhydrous phase cannot be regained upon heating. Aluminum Chloride is used as a Lewis-acid catalyst in Friedel-Crafts reactions where essential products such as detergents, intermediates, and dyes are formed.

Aluminum Chloride is formed when Aluminum metal reacts with Chlorine or Hydrogen Chloride at high temperatures. Dissolution of Aluminum oxides gives hexahydrates.
2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminum Chloride is an irritant and, as such must be handled with care. Some of its properties are given in the table below:
| Name of the molecule | Aluminum Chloride; Aluminum Trichloride |
| No. of valence electrons | (3 x 1) + (7 x 3) = 24 valence electrons |
| Hybridization of the central atom | sp2 |
| Bond Angles | 120° |
| Molecular Geometry of NOCl | Trigonal Planar |
Contents
AlCl3 Lewis Structure
Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. The structure comprises dots and lines to represent electrons and chemical bonds, respectively. The Lewis dot structure of a molecule also helps understand preliminary properties such as molecular geometry, bond angles, and chemical polarity.
Step one in the process of obtaining the Lewis structure is to determine the number of valence electrons present.
Valence Electrons
Valence electrons are those electrons that lie in the outermost shell of the atom. These electrons break free due to weak nuclear attraction and participate in bond formation and electron exchange.
Each atom in the molecule contributes a set number of valence electrons depending upon their atomic number and position on the periodic table. These valence electrons are then used to form the Lewis structure. They are represented as dots.
Aluminum Chloride is a molecule in focus here. It comprises a lone Aluminum atom and three Chlorine atoms. As mentioned above, each Aluminum and Chlorine atom contributes a set number of valence electrons.
Aluminum is in group 13 of the periodic table with the electronic configuration [Ne] 3s²3p¹. Therefore, the lone Aluminum atom in AlCl3 contributes 3 x 1 = 3 valence electrons.
In group 7 of the periodic table, Chlorine has seven valence electrons. Chlorine’s electronic configuration is given by [Ne]3s23p5. The possibility of electrons in its d shell makes it hypervalent. Therefore, the three Chlorine atoms present contributes 7 x 3 = 21 valence electrons.
Therefore, the total number of valence electrons present in Aluminum Chloride (AlCl3) is given by:
3[Al] + 21[Cl] = 24 valence electrons
Lewis Structure Assembly
Twenty-four valence electrons are available as building blocks for the Lewis structure. Next, we form a skeletal structure. Aluminum is the central atom and facilitates bonding with the surrounding Chlorine atoms. This is illustrated in the figure below:

From the 24 valence electrons available, two are placed between each atom to represent chemical bonds. This is shown in the figure below:
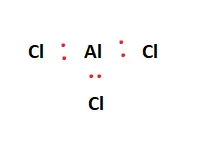
Valence electrons are represented as red dots in the figures attached. Upon forming the chemical bonds within the Lewis structure, the focus shifts to ensure each atom fulfills its outer shell requirements, i.e., adheres to the octet rule. The 18 remaining valence electrons are distributed amongst the atoms to fulfill the aforementioned outer shell requirements.

All valence electrons have been used, and the structural arrangement is now seemingly stable. However, the Aluminum atom in the center only has six valence electrons attached to it. Aluminum does not go on to form double bonds to address this deficiency. It is an exception to the octet rule because it achieves stability with less than 8 electrons attached. We can verify this using the concept of formal charges.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| Al | 3 | 0 | 6/2 | 0 |
| Cl | 7 | 6 | 2/2 | 0 |
| Cl | 7 | 6 | 2/2 | 0 |
| Cl | 7 | 6 | 2/2 | 0 |
Having calculated the formal charges to be zero, we can verify the Lewis structure drawn to be stable. Can you try calculating the standard charges of the molecule when there’s a double bond present? Share your results in the comments below.
Therefore, the final Lewis structure of AlCl3 is shown in the figure below:

AlCl3 Hybridization
Molecular structure and bond formation can be better explained with hybridization in mind. The Hybrid orbitals formed to give a more accurate description of electron regions while also resulting in more stable bonds. An easy way to determine the hybridization of an atom is to calculate the number of electron domains present near it. The bond between atoms (covalent bonds) and Lone pairs count as electron domains.
In this case, the Aluminum atom in Aluminum Chloride forms three sigma bonds with the surrounding Chlorine atoms. This gives the central Aluminum atom a steric number of 3, i.e., three domains are attached to it.
Therefore, the Aluminum atom at the center of the Aluminum Chloride molecule has an sp2 hybridization.
AlCl3 Angles
There are three Chlorine atoms surrounding the central Aluminum atom. According to the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), the Chlorine atoms will repel each other, giving the molecule a Trigonal Planar geometry with bond angles of 120°.
AlCl3 Molecular Geometry and Shape
The Lewis structure of a compound gives insight into its molecular geometry and shape. From the Lewis structure, it can be observed that a set of Chlorine atoms surround the central Aluminum atom.
According to the VSEPR theory, electron regions on atoms will repel each other as much as possible. This repulsion pushes the atoms apart to give molecular geometry. The Chlorine atoms present repel each other to settle into a trigonal planar shape. Aluminum Chloride exists in different states depending on temperature. In this case, the gaseous form of AlCl3, a monomer, is considered for our study.
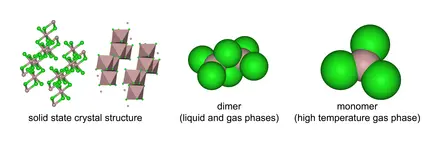
We can use the A-X-N method to confirm this.
‘A’ here represents the central Aluminum atom. Therefore, ‘A’ = 1 in this case.
‘X’ represents the number of atoms bonded to the central atom. In this case, the Aluminum atom forms three covalent bonds with the set of surrounding Chlorine atoms.
Therefore, X =3 for the Aluminum atom.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 0 as there are no lone pairs present.
Therefore, that would give us an A-X-N notation of AX3 for the Aluminum atom and the molecule as a whole.
From the A-X-N table below, we can determine the molecular geometry for Aluminum Chloride (AlCl3).
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
From the above table, it can be observed that an AX3 arrangement corresponds to a Trigonal Planar geometry. Therefore, the Aluminum Chloride molecule possesses a Trigonal Planar molecular geometry and shape.
CONCLUDING REMARKS
Let’s quickly summarize the salient features of Aluminum Chloride
- Exists as a white/pale yellow solid with a distinct odor of HCl. Used in organic synthesis in the alkylation and acylation of arenes.
- ACl3 comprises a single, central Aluminum atom that forms covalent bonds with a set of three surrounding Chlorine atoms.
- The hybridization of the central Aluminum in AlCl3 is sp2.
- Aluminum Chloride has a trigonal planar molecular geometry with bond angles of 120°.