C2H5OH or Ethanol is an organic chemical compound, which can also be represented as CH3-CH2-OH. Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt. It is also used in laboratories for synthesis of other organic compounds and is often stored in wash bottles to use it as a solvent.
| Name of molecule | Ethanol (C2H5OH) |
| No of Valence Electrons in the molecule | 20 |
| Hybridization of C2H5OH | sp3 hybridization |
| Bond Angles | 109° |
| Molecular Geometry of C2H5OH | Tetrahedral |
Now we shall learn about the Lewis structure of this molecule for a better understanding of its physical and chemical properties. The structure helps in further understanding the arrangement of atoms, bond formation and shape of the molecule.

We will first calculate the total number of valence electrons of the molecule, as that will help to comprehend the molecule’s Lewis structure. Let’s get to it then!
Contents
C2H5OH Valence electrons
Valence electrons are the electrons attached to the outer shell of the atom. Here we have three different atoms, C, O, and H.
There are 4 Valence electrons in one Carbon atom. In this compound, we have two atoms of Carbon, so the total number of valence electrons are= 4*2 = 8.
The atom of oxygen has six valence electrons in its outer shell.
Valence electrons for Hydrogen is 1. However, we have six hydrogen atoms, and thus the total number of valence electrons for Hydrogen is = 6*1 = 6.
Hence, we have a total of = 8+6+6 = 20 valence electrons in the Ethanol compound.
C2H5OH Lewis Structure
Now that we know the valence electrons, we can determine the Lewis Structure for this molecule. This structure will help us understand the properties of molecules along with their shape and molecular geometry.
So first, we will determine the central atom for this molecule. Generally, the least electronegative atom takes the central position. Here Carbon is the least electronegative atom. Hence, the central atom in this compound is Carbon, which makes Oxygen and Hydrogen its neighbouring atoms. Hydrogen can share one electron with the other atoms to attain stability as it only needs to have electrons in its outer shell.
Carbon and Oxygen atom needs 8 electrons in its outer shell to fulfil the octet rule, therefore reaching stability. One carbon atom forms four bonds, one with the neighbouring carbon atom and the rest three with hydrogen atoms. This carbon atom has 8 electrons in its outermost shell and is thus stable.
The other Carbon atom also forms four bonds. It includes two single bonds with Hydrogen atoms, one single bond with the neighbouring Carbon atom and one single bond with the Oxygen atom.
As the valence electrons in an Oxygen atom are 6, when oxygen shares two electrons, one each with Carbon and Hydrogen, it is left with two lone pairs of electrons. This is the most stable and accepted Lewis dot structure of Ethanol that we generally use in chemistry.
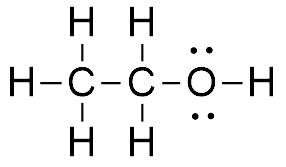
Hence there each Carbon forms four bonds and completes its octet. There are two lone pairs of electrons in this molecule on an Oxygen atom and eight bonding pairs of electrons. Remember to count the bond between the Oxygen and Hydrogen atoms as well.
C2H5OH Hybridization
To find out the hybridization for any molecule, one can either find it out by looking at the number of electron regions or its Steric number. Here as Carbon atoms are in the central position, we will consider its hybridization first. Now each Carbon atom forms four bonds, which means there are four electron regions around each Carbon atom. Hence it needs to form four hybrid orbitals to accommodate these electrons.
Another way to find out the hybridization is to check the number of atoms forming bonds with the atom. Again there are four atoms forming bonds with the Carbon atom, and thus it requires four hybrid orbitals. As a result, it has an sp3 hybridization ( one s orbital and three p orbitals are hybridized to accommodate all these electrons).
Even an Oxygen atom has an sp3 hybridization as it also has four electron regions ( two lone pairs, one bonding pair with Hydrogen and one bonding pair with Carbon).
Thus the overall hybridization of C2H5OH is sp3.
C2H5OH Molecular Geometry
Lewis dot structures for any molecule can only help us with finding the 2D structure. For knowing its 3D structure and the arrangement of the atoms, one needs to know its molecular geometry. Using the AXN Notation method, we can determine the geometry of this molecule.

The notation for this molecule will be AX4 ( A stands for central atom, X is for the number of atoms forming bonds with the central atom, and N is for the number of lone pairs on the central atom). Here Carbon has no lone pairs and forms bonds with four atoms and has a tetrahedral geometry.
C2H5OH Bond Angle

The bond angles in this molecule are approximately 109° as per the VSEPR theory.
C2H5OH Shape
As per the VSEPR theory, a molecule having AX4 notation has a tetrahedral shape and hence Ethanol or C2H5OH, the molecule’s shape is tetrahedral.
Concluding Remarks
To summarize this blog post on Ethanol molecule, we conclude the following:
- C2H5OH comprises two carbon atoms, four hydrogen atoms and a hydroxyl group.
- There are 20 valence electrons for this molecule.
- Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.
- There are two lone pairs of electrons on the Oxygen atom.
- The molecule has sp3 hybridization and a tetrahedral molecular geometry.