The chemical formula ClF3 represents Chlorine Trifluoride. It is an interhalogen compound.
ClF3 is colorless as gas and condenses into a pale green-yellow liquid. The compound is highly reactive, poisonous, and corrosive. Chlorine Trifluoride has been used in a variety of applications since it was first discovered.
The fluorination of Chlorine was first reported to produce ClF3. It reacts with metals to form Chlorine and Fluorine based halides. Examples include Phosphorous Trichloride (PCl3) and Phosphorous Pentafluoride (PF5). It violently reacts with water to give Hydrogen Chloride or Hydrogen Fluoride. Oxygen is also released.
Chlorine Trifluoride is primarily used in the semiconductor industry as a cleaning agent. Its use as a storable oxidizer in rockets has been proposed. However, concerns relating to storage have not been addressed.
ClF3 is hypergolic while also being a very strong oxidizing and fluorinating agent. It is oftentimes incendiary and can cause significant harm if used abhorrently. It is important to take due precautions when dealing with hazardous compounds.
ClF3 has the following properties:
| Name of the molecule | Chlorine Trifluoride (ClF3) |
| No. of valence electrons | 7 + (7 x 3) = 28 valence electrons |
| Hybridization of the central atom | sp3d |
| Bond Angles | 87.5° |
| Molecular Geometry of ClF3 | T-shaped Molecular Geometry |
Contents
ClF3 Valence Electrons
Valence electrons are those electrons that are available for exchanges and bond formation. They are present in the atom’s outermost shell, where the force of attraction from the nucleus is relatively less. This, in turn, makes these electrons readily available upon excitation.
Before jumping into the Lewis structure, we must first determine how many valence electrons are available to us. Each constituent atom in the molecule contributes valence electrons from their outermost shells.
Chlorine Trifluoride comprises three Fluorine atoms and one Chlorine atom.
Being in group 7 of the periodic table, Chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5.
Therefore, the Chlorine atom contributes 7 x 1 = 7 valence electrons.
Fluorine is in group 17 of the periodic table with the electronic configuration [He] 2s22p5. Therefore, the three Fluorine atoms present contribute: 7 x 3 = 21 Valence Electrons.
Therefore, the total number of valence electrons in ClF3 is given by:
7[Cl] + 21[F] = 28 Valence Electrons
ClF3 Lewis Structure
Now that we know the number of valence electrons, we can form covalent bonds and distribute the electrons in accordance with the octet rule.
There are a total of 28 valence electrons available to us. Chlorine acts as the central atom with the Fluorine atoms surrounding it.
We then form covalent bonds between the central Chlorine atom and the surrounding Fluorine atoms using some of the valence electrons available to us.
We then use the remaining valence electrons to fill up the octets of the surrounding Fluorine atoms. This is shown in the figure below.

24 valence electrons have been used. We observe that there are two lone pairs of electrons left over. These two lone pairs attach themselves to the central Chlorine atom as shown in the figure.
This structure looks unusual. To verify its stability, let us calculate the formal charges for the ClF3 molecule.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
|
Element | V | N | B/2 | FC |
| Cl | 7 | 4 | 6/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 |
0 |
The formal charges being 0 for all of the atoms in the molecule tells us that the structure shown above is stable.
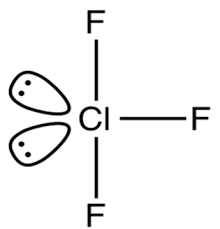
The Lewis Structure for ClF3 is given below:
ClF3 Hybridization
Chlorine Trifluoride comprises 3 Fluorine atoms, all pulled together by the central Chlorine atom. To determine the hybridization, we take a look at the Lewis structure of the ClF3 molecule.
There are two lone pairs on the central Chlorine atom. Chlorine also forms covalent bonds with the surrounding Fluorine atoms.
All in all, there are three Cl-F bonds and two lone pairs of electrons.
To form bonds with Fluorine, the central Chlorine atom requires 3 unpaired electrons.
One 3s orbital, three 3p orbitals, and one 3d orbital participate in the hybridization process. This then leads to the formation of five sp3d orbitals.
Therefore, the hybridization of the central Chlorine atom in Chlorine Trifluoride is sp3d.
ClF3 Bond Angles
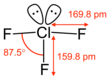
Due to the presence of lone pairs, the constituent atoms repel each other in accordance with the VSEPR theory. The bond angles in ClF3 are 87.5°.
ClF3 Molecular Geometry and Shape
To determine the molecular geometry for Chlorine Trifluoride, we go back to its Lewis structure. From the Lewis structure, it can be observed that Chlorine has an expanded octet. It has two lone pairs attached to it.
There are three Fluorine atoms surrounding the central Chlorine atom as well. We can visualize the same and determine the molecular geometry.

According to the VSEPR theory, the Fluorine atoms will repel each other. Placing the three Fluorine atoms on the central Chlorine atom gives us a Trigonal Planar geometry.
However, upon adding the two lone pairs of electrons, it changes into a T-shaped molecular geometry.
The electronic geometry is Trigonal Bipyramidal.
Therefore, ClF3 has a T-shaped molecular geometry and a Trigonal Bipyramidal electronic shape.
Concluding Remarks
Let’s quickly summarize the salient features of Chlorine Trifluoride
- ClF3 comprises a Chlorine atom surrounded by three Fluorine atoms. There are also two lone pairs of electrons attached to the central Chlorine atom.
- In its most stable state, the Chlorine atom forms three covalent bonds with the surrounding Fluorine atoms.
- The hybridization of the central Chlorine atom in ClF3 is sp3d.
- ClF3 has a T-shaped molecular geometry and a Trigonal Bipyramidal electronic shape with bond angles of 87.5°.