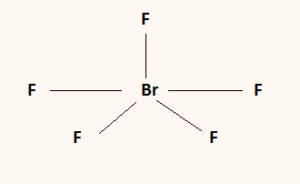
The chemical formula BrF5 represents Bromine Pentafluoride. It is an interhalogen compound and a strong fluorinating agent. Interhalogen compounds exclusively comprise two or more different halogen atoms.
BrF5 is prepared by treating Bromine with large amounts of Fluorine at temperatures of over 150°C. It reacts violently with water but produces Bromic Acid and Hydrofluoric Acid upon dilution.
It finds use in analytical studies and practical applications. Being a strong fluorinating agent, it reacts with Uranium to form Uranium Hexafluoride. This is then used to enrich Uranium.
Bromine Pentafluoride is extremely hazardous and must be handled with care. It is extremely corrosive to the skin and can ignite spontaneously.
BrF5 has the following properties:
| Name of the molecule | Bromine Pentafluoride (BrF5) |
| No. of valence electrons | 7 + (7 x 5) = 42 valence electrons |
| Hybridization of the central atom | sp3d2 |
| Bond Angles | 90° |
| Molecular Geometry of BrF5 | Square Pyramidal Molecular Geometry |
Contents
BrF5 Valence Electrons
The electrons present in the outermost shell of the atom are the ones that are available for bond formation and exchange. This is because the force of attraction from the nucleus is weakest in the outermost shells.
These electrons are called valence electrons. To draw the Lewis structure for a compound, we must first calculate the number of valence electrons available to us. Each element in the compound contributes a certain number of valence electrons from its outermost shell.
BrF5 is an interhalogen compound that comprises Bromine and Fluorine. There are five Fluorine atoms and one Bromine atom.
Bromine is in group 17 of the periodic table and has an electronic configuration of [Ar] 4s²3d¹⁰4p⁵. Therefore, the Bromine atom contributes: 7 x 1 = 7 Valence Electrons
Fluorine is in group 17 of the periodic table with the electronic configuration [He] 2s22p5. Therefore, the five Fluorine atoms present contribute: 7 x 5 = 35 Valence Electrons.
Therefore, the total number of valence electrons in BrF5 is given by:
7[Br] + 35[F] = 42 Valence Electrons
BrF5 Lewis Structure
The Lewis structure of a compound represents a schematic arrangement of all the atoms present in the compound. It tells us about the nature of the bonds present while also giving insight into the molecular geometry and bond angles of the compound.
The outermost electrons are also shown attached to their constituent atoms as well. Lewis structures also help predict polarity and reactivity.
The first step to determining the Lewis structure is to calculate the number of valence electrons available. There are 42 valence electrons available to us. We can now use these to form bonds and fill octets.
Bromine acts as the central atom here and will facilitate bond formation. Bromine forms covalent bonds with the surrounding Fluorine atoms. This is represented in the figure below. Two valence electrons are used in the formation of a Bromine- Fluorine bond.
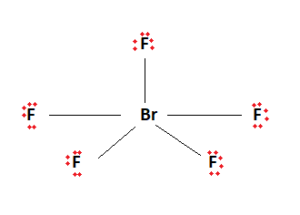
Ten valence electrons have been used. The remaining valence electrons are then used to fill the outermost shells of the constituent elements. We fill up the outermost shells of the surrounding Fluorine atoms.
The two remaining valence electrons act as a lone pair. This lone pair is then attached to the central Bromine atom. We are now left with the final Lewis structure of BrF5.
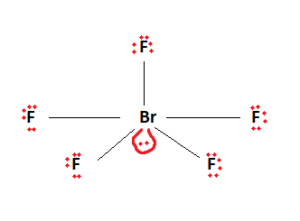
It is observed here that the central atom, Bromine, has 12 valence electrons attached to it. Being in period 4 of the periodic table, it is capable of having this expanded octet.
To verify the Lewis structure shown above, we can calculate its formal charges.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
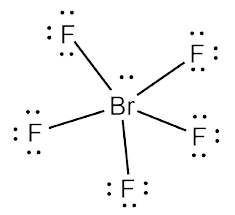
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| Br | 7 | 2 | 10/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
The formal charges being 0 for all of the atoms in the BrF5 molecule tells us that the structure shown above is stable.
BrF5 Hybridization
Bromine Pentafluoride comprises 5 Fluorine atoms, all pulled together by the central Bromine atom. To determine the hybridization, we take a look at the Lewis structure of the BrF5 molecule.
It can be observed from the BrF5 Lewis structure that there are five Fluorine atoms surrounding the central Bromine atom. There is also a lone pair attached to the Bromine atom.
The electronic configuration of the Bromine atom is given by 1s2 2s22p6 3s23p63d104s24p5. To make itself available for bonding, some of the electrons are shifted to the 4d-orbitals. Two of the 4p-orbitals become unpaired and Bromine enters an excited state, making it available for hybridization.
One 4s, three 4p and two 4d orbitals take part in the process giving rise to sp3d2 hybrid orbitals. Sigma bonds are formed between Bromine and Fluorine.
Therefore, the hybridization of the central Bromine atom in BrF5 is given by sp3d2.
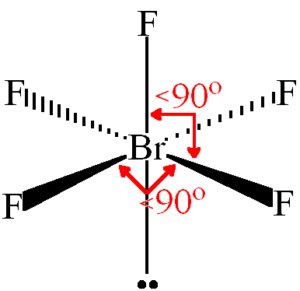
BrF5 Bond Angles
The constituent atoms repel each other in accordance with the VSEPR theory. The bond angles in BrF5 are 90°.
BrF5 Molecular Geometry and Shape
To determine the molecular geometry for Bromine Pentafluoride, we go back to its Lewis structure.
From the Lewis structure, it can be observed that Bromine has an expanded octet. It also forms five singular covalent bonds with the surrounding Fluorine atoms. There is also one lone pair attached to the central Bromine atom.
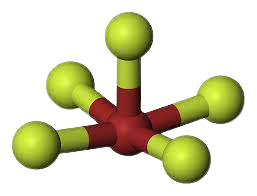
Considering the above, the Fluorine atoms repel each other and give the molecule a Trigonal Bipyramidal shape. Upon the addition of the lone pair, however, the Fluorine atoms get pushed in further. This gives the molecule a Square Pyramidal shape.
Therefore, BrF5 has a square pyramidal molecular geometry and an Octahedral electronic shape.
Concluding Remarks
Let’s quickly summarize the salient features of Bromine Pentafluoride
- BrF5 comprises a Bromine atom surrounded by five Fluorine atoms. There’s also a lone pair of electrons attached to the central Bromine atom.
- In its most stable state, the Bromine atom forms five singular covalent bonds with the surrounding Fluorine atoms.
- The hybridization of the central Bromine atom in BF5 is sp3d2.
- BrF5 has a Square Pyramidal molecular geometry and an Octahedral electronic shape with bond angles of 90°.