We have learnt Fe Configuration before. The Tellurium element is a semi-metal found in tiny amounts in the earth’s crust. It is a group 16 element with the chemical symbol of Te.
Tellurium electron configuration
It has an atomic number of 52. To find out the electron configuration of an atom, we first need to know the total number of electrons. So here as this element has 52 atomic numbers, it means the element has 52 electrons. Once we know the number of electrons, we can start arranging the electrons in the orbitals.
Tellurium electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4
Or
[Kr] 5s2 4d10 5p4
For writing the electron configuration, here are some general chemistry rules that you should know:
We use several theories and rules such as Aufbau Principle, Hund’s rule and the Pauli-Exclusion principle to write the electron configuration. Each orbital can have two electrons of the opposite spin, and depending upon its level, it can occupy the possible number of electrons in each orbital in a given subshell.
| Subshell | Number of orbitals | Total number of electrons in the orbital |
| s | 1 | 2 |
| p | 3 (px, py, pz) | 6 |
| d | 5 ( dx2-y2, dz2, dxy, dxz, dyz) | 10 |
| f | 7 (fz3, fxz2,fxyz,fx(x2-3y2), fyz2,fz(x2-y2), fy(3×2-y2) | 14 |
There are four types of subshells which are: s, p, d and f.
Each s subshell can take up to 2 electrons in its shell.
Each p subshell can accommodate 6 electrons in its electron shell.
Each d subshell can take up to 10 electrons in its electron shell.
An f subshell can have up to 14 electrons in its shell.
For writing the electron configuration, we use the Aufbau Principle or which is also called the building up principle. It states the electrons occupy the orbitals in the order of increasing energy. Meaning it will first occupy the orbitals with less energy and then fill the higher energy orbitals.
The order of occupation for electrons is:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p
One can also guess the order of electrons for any element by following Madelung’s rule:
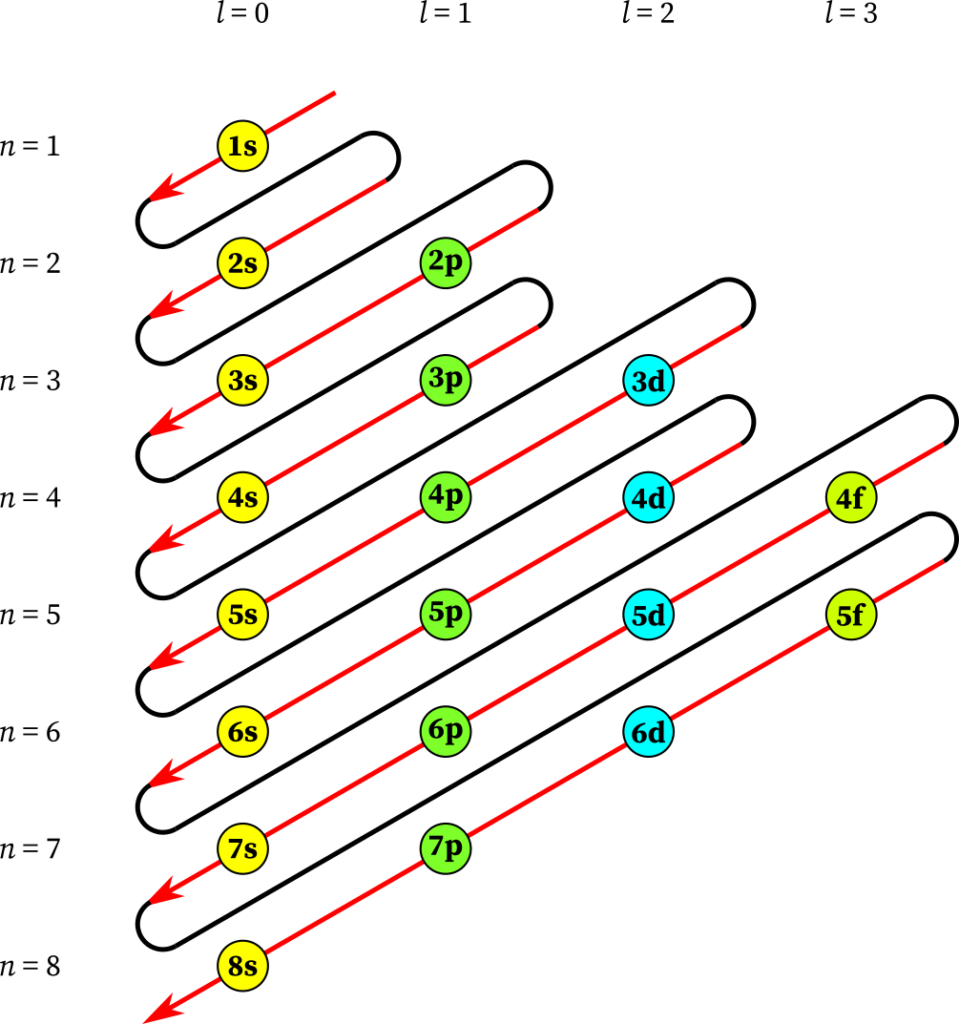
Now here as we have 52 electrons for Tellurium, the order of occupation will be 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4
Tellurium Properties:
| Property | |
| Chemical name | Tellurium |
| Atomic weight | 127.6 u |
| State | Solid ( at 20℃) |
| Boiling point of Tellurium | 1261 K |
| Melting point of Tellurium | 722.66 K |
| Color of Tellurium | Silver lustrous gray ( crystalline), brown-black powder (amorphous) |
| Density | 6.232 g/cm3 ( at RT) |
| Solubility | Not soluble in water and most acids |
Tellurium Uses
- Tellurium is widely used in making alloys with other elements such as copper, stainless steel and iron.
- As it is resistant to strong acids, Tellurium provides the alloy with better strength, durability and resistance against acids and corrosion.
- It is also used to make cadmium telluride solar cells.
- When used along with lead, it forms Lead telluride that is used as an infrared sensor.
- Tellurium is also used to color the ceramics and in the making of the fiberglass that is used in telecommunications.
- The element is used as a catalyst for oil refining and is used in semiconductor applications.
However, Tellurium has no biological role and is toxic. People exposed to very small Tellurium in the air develop “tellurium breath” with a garlic-like odor.