Silicon is a chemical element with the atomic number Z = 14. It can be found under Carbon (Z = 6) in the periodic table of elements and is commonly classified as a metalloid. Silicon appears as a dark blue-grey solid with a metallic luster. It is a hard, brittle crystalline solid. Silicon binds with oxygen to form silicates commonly found in the earth’s crust.
Silicon is relatively inert but reacts vigorously with halides and metals at high temperatures. Silicon forms a continuous layer of SiO2 over the metallic surface to protect it from Oxidation. Silicon also reacts with Carbon and hydrogen to form silicon carbide (SiC) and hydrides.
Most Silicon used commercially is unprocessed; however, Silicon has played a key role in ushering in the digital age we know today. High-grade pure Silicon is infused with boron, arsenic, and phosphorus to produce silicon wafers used in transistors, diodes, liquid crystal displays, and integrated circuitry. Other silicon-based compounds and minerals, such as silica and quartz, are used in construction and glassware.
Let us dive deeper into its electron structure in the next section.
Electronic Configuration
Silicon’s electronic structure makes it an extremely valuable semiconductor. Adding donor and acceptor atoms in the crystalline silicon lattice allows for positive and negative types of extrinsic conduction. Semiconductor electronics are a multi-billion dollar industry.
Scientist Niels Bohr first described electron structures and orbits in 1913. Electrons in the atom revolve around the nucleus in paths known as orbits. Orbits can be expressed using the symbol n [n = 1,2,3,4,…]. The first four orbits are labeled K, L, M, and N, where n = 1,2,3 & 4, respectively. The electron capacity of each orbit is given by 2n2. Ex. The number of electrons in K orbit = 2(1)2 = 2. Here n=1.
Then, the electronic configuration of an element describes how its electrons are distributed in its orbitals. For Silicon, atomic number Z = 14. The 14 electrons can be distributed amongst the first three atomic shells in a 2,8,4 configuration.
The electronic configuration of Silicon can also be expressed as a function of orbital sub-energy levels determined by the Azimuthal quantum number. These are based on the Aufbau principle and are represented by s,p,d, and f.
The orbit number n can be used to determine the Azimuthal number ‘I.’ ‘I’ ranges from 0 to n-1. In this system, the s-energy shell has one orbital; the p-energy shell has three orbitals, and so on, up to the f-energy shell, which has seven orbitals. Each orbital can hold up to 2 electrons.
| Orbit Number | Value of ‘l’ | Number of subshells | Number of orbitals | Subshell name | Electrons holding capacity | Electron configuration |
| 1 | 0 | 1 | 1 | 1s | 2 | 1s2 |
| 2 | 01 | 2 | 13 | 2s2p | 26 | 2s2 2p6 |
| 3 | 012 | 3 | 135 | 3s3p3d | 2610 | 3s2 3p6 3d10 |
| 4 | 0123 | 4 | 1357 | 4s4p4d4f | 261014 | 4s2 4p6 4d10 4f14 |
The Aufbau principle shown in the diagram below is used to fill in the orbitals with electrons. We go from 1s to 2p to 2s and so on. Again, remember that each orbital can hold 2 electrons and p subshells have three orbitals present.

In the case of Silicon, Z =14. So the first two electrons in Silicon will go to the 1s subshell. The next two will go to the 2s subshell. This process eventually gives us the electron configuration: 1s2 2s2 2p6 3s2 3p2. The arrangement can be represented by orbital boxes, with the arrows inside representing individual electrons.
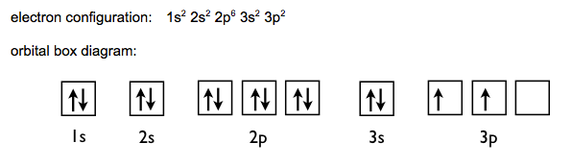
In conclusion, Silicon has an electronic configuration of 2,8,4 and an orbital configuration of 1s2 2s2 2p6 3s2 3p2.