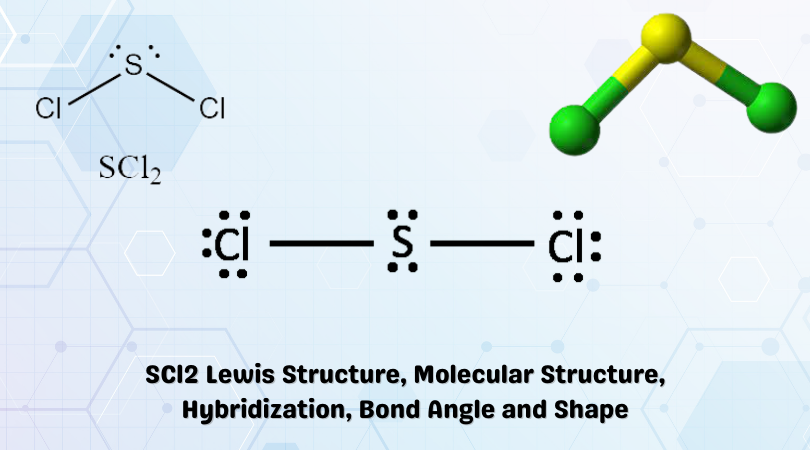
The chemical formula SCl2 represents Sulfur Dichloride. It is the simplest form of Sulfur Chloride and exists as a cherry-red liquid at room temperature. It is obtained via chlorination of S2Cl2 whose impure presence is then distilled using PCl3 to give pure Sulfur Dichloride. It is a corrosive agent and is hazardous to the environment. SCl2 reacts with alkenes and ethylene to form organic thioether (sulfide) compounds. One example is the dangerous Sulfur Mustard used in chemical warfare. The properties of SCl2 are as follows:
| Name of the molecule | Sulfur Dichloride (SCl2) |
| No. of valence electrons | 6 + (2 x 7) = 20 valence electrons |
| Hybridization of central atom | sp3 |
| Bond Angles | 103° |
| Molecular Geometry of SCl2 | Bent Molecular Geometry |
Contents
SCl2 Valence Electrons
Sulfur is in group 6(Chalcogens) of the periodic table with the electronic configuration [Ne] 3s²3p⁴. Therefore, the Sulfur atom contributes 6 x 1 = 6 valence electrons Being in group 7 of the periodic table, Chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5. The possibility of electrons in its d shell makes it hypervalent. Therefore, the two Chlorine atoms contribute 7 x 2 = 14 valence electrons. Now, the total number of valence electrons available in SCl2 is given by: 6[S] + 14[Cl] = 20 valence electrons.
SCl2 Lewis Structure
Sulfur is the least electronegative and therefore, is placed in the center of the skeletal structure. We then line up the two Chlorine atoms on either side of the Sulfur atom. We will now begin placing the 20 valence electrons available to us in accordance with the octet rule.
The electrons are first placed in between the atoms to form a covalent bond between Sulfur and the two Chlorine atoms as shown in the figure. The remaining valence electrons are filled in the outermost atoms, gradually moving inward. We place 6 electrons on each of the two Chlorine atoms as shown in the figure.
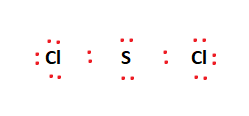
This leaves us with four remaining valence electrons. These electrons are placed as lone pairs on the Sulfur atom in the center of the molecule as shown in the figure. Therefore, the final Lewis structure of the SCl2 molecule is given below:
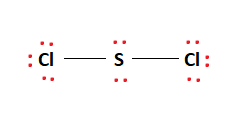
It can be seen in the figure above that the octet rule is satisfied completely. This makes the above Lewis structure fully stable.
SCl2 Hybridization
Sulfur Dichloride comprises two Chlorine atoms separated by a lone Sulfur atom. This Sulfur atom forms two covalent bonds with each of the Chlorine atoms. The fulfillment of the octet rule on each of the Chlorine atoms leaves 4 valence electrons. These act as lone pairs and attach themselves on opposite ends of the Sulfur atom. This gives us four electron domains- two covalent bonds and two lone pairs. Therefore, the hybridization of the molecule is sp3.
SCl2 Bond Angles
According to the VSEPR theory, the Chlorine atoms and the lone pairs repel each other. This gives SCl2 a bond angle of 103°.

SCl2 Molecular Geometry and Shape
As seen in the Lewis structure above, the Chlorine atoms repel each other. This gives us a linear shape initially. However, upon the addition of the two lone pairs on Sulfur, the molecular geometry becomes bent. This is in accordance with the VSEPR theory.

Therefore, SCl2 has a Bent molecular geometry and a tetrahedral shape in nature.
Concluding Remarks
Let’s quickly summarize the salient features of SCl2
- SCl2 consists of a single Sulfur atom surrounded by two Chlorine atoms.
- In its most stable state, Sulfur acts as the central atom and forms two covalent bonds with the Chlorine atoms. It also possesses two lone pairs.
- Due to the presence of 4 electron domains and its steric number being 4, the hybridization of SCl2 is given by sp3.
- SCl2 has a bent molecular structure and a tetrahedral electronic shape.
- It has bond angles of 103°.