Dinitrogen Difluoride is an inorganic compound having a chemical formula of N2F2. It is one of the most stable and strong halides of Nitrogen. The compound exists as a gas a the standard room temperature.
| Name of molecule | Dinitrogen Difluoride ( N2F2) |
| No of Valence Electrons in the molecule | 24 |
| Hybridization of N2F2 | sp2 hybridization |
| Bond Angles | 118° |
| Molecular Geometry of N2F2 | Trigonal pyramidal |
A unique fact about this compound is that it exists in both cis and transforms. In this blog post, we will look at the total number of valence electrons for this molecule, followed by its Lewis Structure, Hybridization, and more.
Contents
N2F2 Valence electrons
The electrons that are present in the outermost shell of the atom are known to participate in bond formation and are known as valence electrons. The electrons are vital as it helps to form the structure of the molecule. To understand the Lewis Structure of any compound, we always check the total number of valence electrons first.
Here Nitrogen has five valence electrons, but as there are two Nitrogen atoms here, we will multiply the number by 2.
Fluorine has seven valence electrons. We will multiply this number by two as well, as there are two Fluorine atoms here.
Total number of valence electrons in N2F2 – valence electrons of Nitrogen + valence electrons of Fluorine
= 5*2 + 7*2
= 10 + 14
= 24 valence electrons
Thus, N2F2 has a total of 24 valence electrons.
N2F2 Lewis Structure
The Lewis structure of any molecule or compound helps us understand valence electrons’ arrangement in the structure. It is a pictorial representation of the electrons forming bonds and the ones that do not participate in any bond formation for the given molecule or compound.
The bonds formed between the atoms are shown by drawing straight lines, whereas the lone pairs or nonbonding pairs of electrons are represented by using dots.
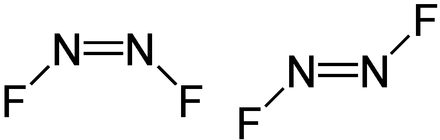
For N2F2, both the Nitrogen atoms are placed in the centre as it is less electronegative than the Fluorine atoms. The Fluorine atoms are placed on the sides of these Nitrogen atoms like this:
If you look at the Fluorine atoms, it just requires one electron to complete its octet. To attain a stable structure, both these Fluorine atoms will share one electron each of Nitrogen atom. Hence both Nitrogen atoms form a single bond with Fluorine atoms, and four valence electrons out of 24 are used up.
To show the complete octets for Fluorine, arrange seven electrons around each Fluorine atom, which means we have now used up 7*2+4= 18 electrons out of 24.
Lastly, we try to complete the octets of the Nitrogen atoms. Both these atoms have four valence electrons each after sharing one with the Fluorine atom. The nitrogen atom shares a double bond to try and complete its octet. These double bonds use up four more valence electrons. As a result, each Nitrogen atom is left with one lone pair of electrons.
So, N2F2 has one double bond between both the Nitrogen atoms and two single N-F Bonds along with two pairs of nonbonding pairs of electrons.
N2F2 Hybridization
To find out the Hybridization of this molecule, we will calculate the total number of electron density regions and using the table; we will find out its Hybridization.
Here we look at the Hybridization of the Nitrogen atom as it forms bonds with other Nitrogen atom and Fluorine atoms. So for a single Nitrogen atom, there are a total of 3 electron density regions which comprises of:
- One lone pair
- N-F bond
- And a double bond with the neighbouring Nitrogen atom.
So as there are three electron density regions for the Nitrogen atom, it has sp2 Hybridization. One can also apply other formulas of finding out Hybridization that we shared previously. This will hold true for the other Nitrogen atom as well. Hence the Hybridization for N2F2 is sp2.

N2F2 Bond angles
The electron arrangement in N2F2 is trigonal planar, as the central Nitrogen atom is forming bonds with two other atoms. Generally, the bond angles for the molecules having such an arrangement is 120°, but as there are two lone pairs, it will try and repel each other, compressing the bond angle to 118°.
N2F2 Molecular Geometry
The molecular geometry of Dinitrogen Difluoride is trigonal planar as the electrons are arranged in the trigonal planar geometry.
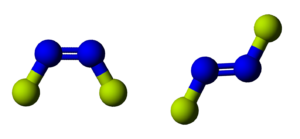
N2F2 Shape
As mentioned earlier, the molecule exists in two forms – cis and trans. Although its trans isomer is much stable, we consider both these structures while talking about the shape of the molecule. It has a trigonal pyramidal as there are lone pairs on the central atom. Hence, N2F2 has a trigonal pyramidal shape.
Concluding Remarks
To summarize this blog post on N2F2, we can say the following:
- N2F2 is made of two Nitrogen and two Fluorine atoms.
- There is one double bond between both the Nitrogen atoms.
- Each Nitrogen atom forms a pi bond with fluorine atoms.
- There are two lone pairs of electrons in the structure.
- The central Nitrogen atom has sp2 Hybridization with a bond angle of 118°.
- It has a trigonal planar molecular geometry and trigonal pyramidal molecular shape.