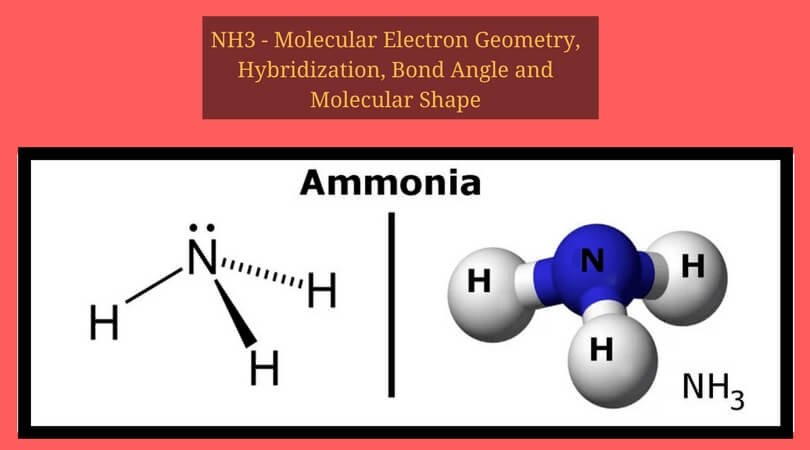
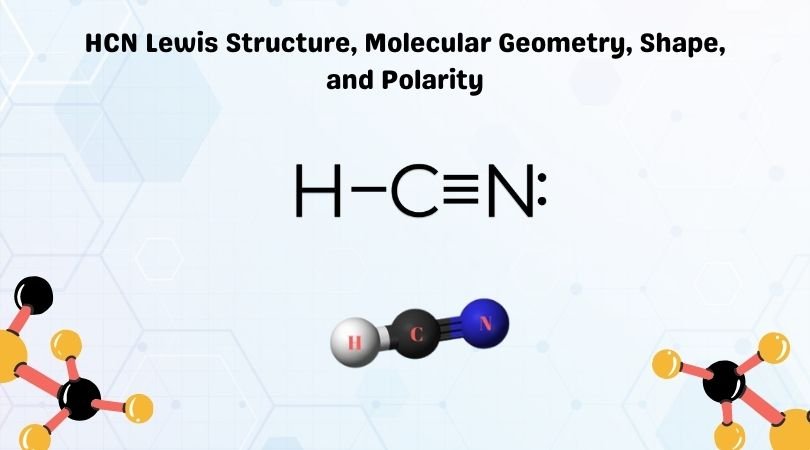
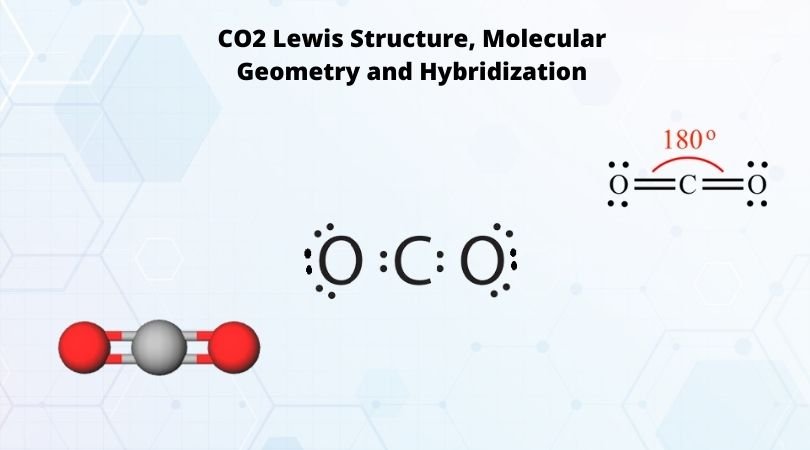
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.
| Name of molecule | Phosphorus trichloride ( PCl3) |
| No of Valence Electrons in the molecule | 26 |
| Hybridization of PCl3 | sp3 hybridization |
| Bond Angles | Less than 109 degrees |
| Molecular Geometry of PCl3 | Trigonal Pyramidal |