Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is the base anhydride for NaOH, as when Na2O reacts with water, it produces NaOH.
Na2O + H2O — 2NaOH
The compound is widely used in ceramics and glasses. Also, this molecule is different from the other organic molecules as it is made up of one Metal ( Sodium) and one non-metal (Oxygen). Such compounds are known as ionic compounds, as there are ionic bonds formed in such molecules. Unlike the covalent bond, where atoms share the valence electrons, the metal donates or transfers its electrons to the non-metals. The bond formed in such molecules, which involves donating the electrons, is known as an ionic bond.
To understand this molecule better, let’s go through its Lewis Dot Structure, Properties and Uses.
| Property | |
| Compound name | Sodium Oxide |
| Molar mass of Na2O | 61.97 g/mol |
| State | Crystalline solid |
| Boiling point of Na2O | 1950 ℃ |
| Melting point of Na2O | 1132 ℃ |
| Color of Na2O | White |
| Density | 2.27 g/cm3 |
| Solubility | Reacts with water and ethanol |
Contents
Na2O Valence electrons
For determining the Lewis structure of any molecule, one needs to know the valence electrons for that molecule. Here the metal oxide consists of two Sodium atoms and one oxygen atom.
Sodium is a group 1 element and has one valence electron in its outer shell.
Oxygen is a group 6 element and has six valence electrons in its outer shell.
Na2O Lewis Structure
As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Unlike the standard process of determining the central atom and arranging the electrons around it, we follow it differently.!
For Na2O Lewis Structure, place two Sodium atoms using its Chemical Symbol and its valence electron.
Now place one Oxygen atom with its valence electrons near the Sodium atom.
If you look closely, Oxygen needs two valence electrons to have a complete octet. And these two electrons will be donated by the two Sodium atoms here. Transfer the one valence electron on Sodium atoms to the Oxygen atom.
After transferring electrons, Oxygen has a complete octet, and it also acquires charges as it is accepting electrons. As the Oxygen atom accepts two valence electrons, it will acquire a 2- charge. So put brackets around the Oxygen atom and mention the charge on it.
Similarly, each Sodium atom will acquire a positive charge as it is giving away one electron. Place brackets and place a 1+ charge on each Sodium atom. The net charges on this molecule will be zero. And this is how you can write the Lewis Structure for Na2O.
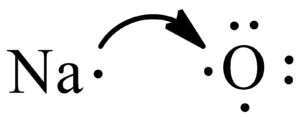
Now, remember that this is just a way to represent the arrangement of electrons for understanding the structure. And this is only one unit that keeps repeating in the entire motif to form a crystal. Ionic compounds have a crystal structure that comprises repetitive motifs.
Preparation of Sodium Oxide
The metal oxide of sodium is prepared by reacting Sodium with Sodium Hydroxide.
2Na + 2NaOh — H2 + 2Na2O
Alternatively, Na2O is also a product of the reaction between sodium nitrite and sodium.
6Na + 2NaNO2 — N2 + 4Na2O
It is also produced by using Sodium peroxide or decomposing the sodium salt of ascorbic acid over 209 ℃.
Na2O Chemical Properties
Sodium Oxide reacts with Carbon dioxide and produces Sodium Carbonate.
It also reacts with some acids such as Hydrochloric acid and produces Sodium Chloride and water.
Although Sodium oxide is not soluble in water, it reacts violently when exposed to water and forms Sodium Hydride.
Na2O Uses
Sodium Oxide is widely used as a component of glass ( Sodium Carbonate) in the form of “Soda”.
It is used in making ceramics, clay bowls, etc.
To learn more about such chemical compounds, let us know which other chemical compound’s structure and properties you would like to know in the comments below. Keep Learning!