The chemical formula SO2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick.
A large quantity of SO2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect.
Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components (Sulfide ores, Sulfites) in the presence of Oxygen. The gas released is captured and primarily used in the production of Sulfuric Acid through the contact process. Here, SO2 is converted to Sulfur Trioxide, which combines with sulfuric acid to give Oleum (disulfuric acid). A combination of Oleum with water gives Sulfuric Acid. Billions of kilograms of SO2 are produced annually to meet global requirements.
In addition to its wide industrial use, SO2 is also used as a preservative in dried fruits, a reagent in the laboratory, and various biomedical applications. It is also being looked at as a potential refrigerant and climate engineering tool.
The chemical polarity of Sulfur Dioxide will be discussed below. We will look at its electronegativity, its molecular geometry and observe if there are any dipoles present.
Contents
Electronegativity and Bond Nature
A difference in electronegativity creates dipoles whereby electronegative forces dictate heavily. This makes the nature of the bond polar.
We look to the periodic table to determine the difference in charges between the Sulfur and Oxygen atoms. In this case the difference is 3.44 [O] – 2.58 [H] = 0.86.
This tells us that the Oxygen atoms are slightly more electronegative than the Sulfur atom, which is indicative of polar nature.
Now, we look to the Pauling scale to help verify. According to the Pauling scale, if the difference electronegativity lies between 0.5 and 2.0, the bonds are polar in nature.
Since that is the case above (0.86), we can safely say that the S-O bonds are polar.
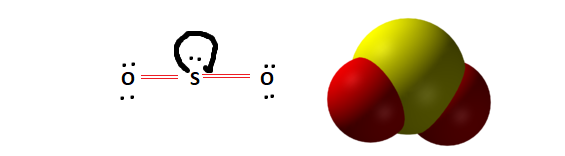
Molecular Shape and Symmetry
Observing the Lewis structure and molecular shape will further help us determine whether SO2 is polar or non-polar. From the Lewis structure, it is evident that the molecule is vertically symmetrical. However, the presence of two lone pairs creates an imbalance in electronic distribution. This lone pair of electrons distorts the molecular geometry giving SO2 its Bent Molecular shape.
The presence of a lone pair indicates that the SO2 molecule is indeed polar.
Net Dipole Moment
There is a net dipole moment present due to the lone pair on Sulfur and its bent shape. This is also due to its bent molecular structure.
The red regions indicate electropositivity in the figure above, while the blue areas are a little more electronegative. This suggests that there are two poles which means that SO2 is a polar molecule.
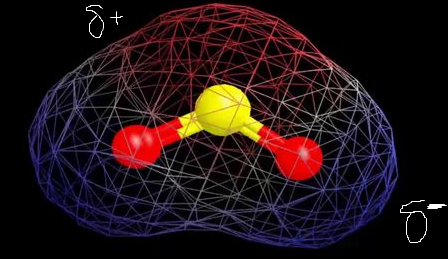
So, SO2 Polar or NonPolar?
Having calculated the difference in electronic potential, we know that the Oxygen-Sulfur bond is polar. Furthermore, the presence of a lone pair on the central sulfur atom and bent shape means that there is a lack of symmetry.
This then creates relatively more electronegative regions near the oxygen atoms. There is a net dipole moment, and thus the molecule is a polar one.
Therefore, SO2 is a polar molecule.