Is H2O a polar molecule?
Yes, H2O or water is a polar molecule and a polar solvent as well. The molecule is made up of two hydrogen atoms and one oxygen atom. To understand why this molecule is polar and nonlinear keep reading this article.
When you look at the water molecule’s molecular geometry, you will notice two lone pairs of electrons on an Oxygen atom that lead to the nonlinear geometry. As there are repulsive forces between lone pairs of electrons, it pushes the Hydrogen atoms downwards.
Both the Hydrogen atoms take up a position like the one shown in the figure to keep the repulsive forces at the minimum. Due to this arrangement of both Hydrogen atoms the shape of the molecule is bent making the geometry of this molecule nonlinear.
Why is water a polar molecule?
- The polarity of any molecule depends on these following factors:
- Its shape
- the difference of electronegativities between the atom and
- the net dipole moment in the molecule.
Here Oxygen atom has the electronegativity of 3.44 whereas the Hydrogen has an electronegativity of 2.20. If you calculate the differences between Oxygen and Hydrogen’s electronegativities, it is much higher than 0.5, making the O-H bond a polar covalent bond.
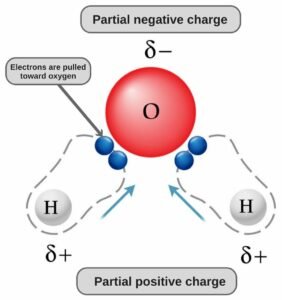
And as there is a difference of electronegativity between both the atoms, there will be dipole moment towards the Oxygen atom. In this molecule, Oxygen is more electronegative and will try to pull the shared electrons towards itself. As a result of this pull, the dipole moment’s direction will be from Hydrogen towards the Oxygen atom on both sides.
As a result of the dipole moment on both sides, there is a net dipole moment in this molecule. This would have been avoided if the shape of the molecule was linear. But as the shape of the molecule is bent, the opposite dipole moments are not cancelled, which means there is a net dipole moment in the molecule.
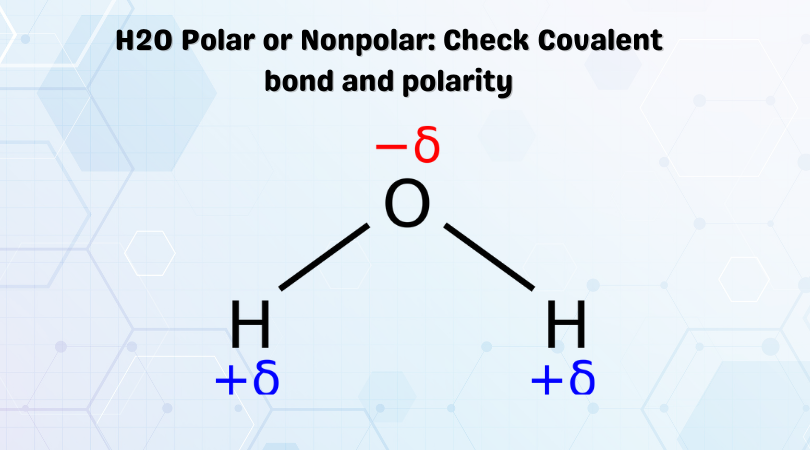
This dipole moment leads to the uneven distribution of charges in the molecule. The region around Hydrogen atoms will have partial positive charges, and the region around the Oxygen atom will have partial negative charges. Due to the formation of these poles in this molecule, H2O is a polar molecule.
Now you’d be astonished to know, but water molecules have a net change of 0. Although there are poles in the molecules and a net dipole moment, it is an electrically neutral molecule. Each water molecule is made up of 10 protons, 10 electrons and a zero net charge.
Water as a polar solvent
Water is a unique molecule that can exist in different states and interact with other water molecules and other elements given it is also a polar solvent. The molecule of water can get attracted to either positive charges or the negative charges of the solute. This happens because the charges near the Oxygen atom are slightly negative and is drawn towards the positive-charged regions of the elements. Similarly, the areas around Hydrogen atoms are positively charged and are attracted to the negatively-charged parts of the molecule.
And this concept is also applied when it also forms bonds with other water molecules. There are weak Hydrogen bonds between water molecules. Although these bonds can hold the water molecules together, they are not as strong as the covalent bonds.



very nice
Thank you for the kind words!