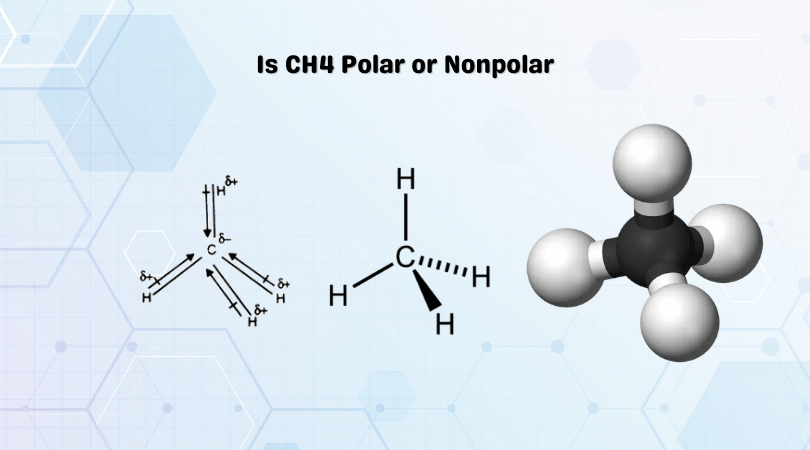
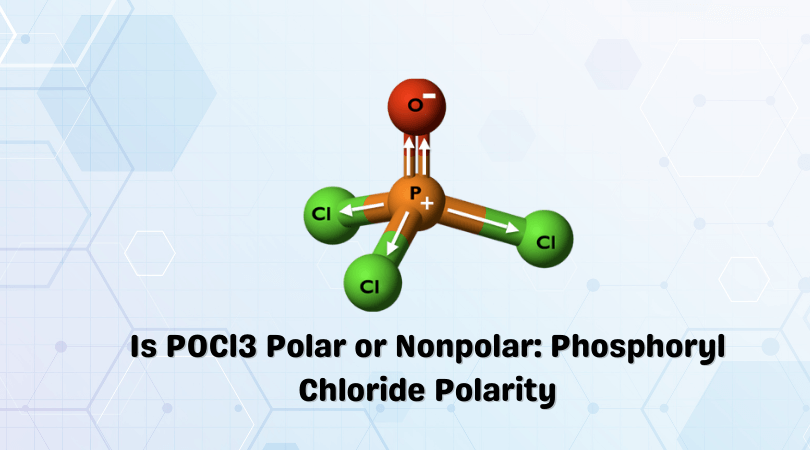
Methane or CH4 is a nonpolar molecule. To find out the reason why the molecule is nonpolar, let’s go through the factors that help us determine the polarity of the molecules.
You can also refer to the CH4 Lewis structure first to understand the arrangement of atoms in the molecule along with its properties. Here we are going to look at some of the parameters that can affect the polarity of the molecule.
The difference in electronegativities of the atoms in the molecule
the CH4 molecule, two atoms are participating in bond formation – Carbon and Hydrogen. The electronegativity of the Carbon atom is 2.5, and the electronegativity for Hydrogen atoms is 2.1
If you calculate the difference, 2.5-2.1, it comes to 0.4
The atoms have an electronegativity difference of 0.4, and both these atoms share a nonpolar bond.
The symmetry of the molecule

Methane molecule have a symmetric shape as all the four Hydrogen atoms are arranged around Carbon atom in the symmetry. Apart from this, all atoms share one valence electron each of the Carbon atom. This overall symmetry will nullify the dipole moments in the molecule. Here as Carbon is more electronegative, it will try and pull electrons to itself. But as the shape is symmetric for this molecule, all the dipole moments cancel out each other.
Net Dipole moment
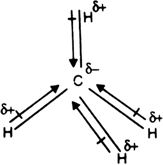
The net dipole moment in the molecule is the sum of the dipole moments that exist in the molecule. Here as all the dipole moments are canceled out, there is zero dipole moment in the CH4 molecule. And as there is no net dipole moment in the molecule, there are no poles formed for CH4, making methane a nonpolar molecule.
Concluding Remarks
Methane or CH4 molecule is a nonpolar molecule as there is no net dipole moment in the molecule.